Factors affecting the fate of β-carotene in the human gastrointestinal tract: A narrative review
Abstract
Abstract. Carotenoids and their metabolites play crucial roles in human health such as in immunity, cell differentiation, embryonic development, maintenance of plasma membrane integrity, and gastrointestinal functions, in addition to counteracting night blindness and other eye-related diseases. However, carotenoid bioavailability is highly variable and often low. The bioavailability of β-carotene, among the most frequently consumed carotenoid from the diet, is determined by food matrix related factors such as carotenoid dose, its location in food the matrix, the physical state in food, the presence of other food compounds in the matrix such as dietary fiber, dietary lipids, other micronutrients present such as minerals, and food processing, influencing also the size of food particles, and the presence of absorption inhibitors (fat replacers and anti-obesity drugs) or enhancers (nano-/micro-formulations). However, also host-related factors such as physiochemical interactions by gastrointestinal secretions (enzyme and salts) and other host-related factors such as surgery, age, disease, obesity, and genetic variations have shown to play a role. This review contributes to the knowledge regarding factors affecting the bioavailability of β-carotene (food and host-relegated), as well as highlights in vitro models employed to evaluate β-carotene bioavailability aspects.
Introduction
Vitamin A deficiency is one of the most common micronutrient deficiency disorders in the modern world, mostly affecting developing countries with low animal-derived products and thus low preformed vitamin A intake. Worldwide, more than 250 million preschool children are estimated to suffer from vitamin A deficiency [1, 2]. The term carotenoids is the generic name for the members of a family of compounds containing typically 40 carbon atoms, though C-30 and C-50 carotenoids from bacteria and fungi have also been reported [3]. Major classes include carotenes (carotenoids without oxygen function) or xanthophylls (carotenoids including oxygen in their molecule) [4]. Approximately 50 carotenoids have been reported in the human diet [5], and three major carotenoids which can be converted in vivo into vitamin A include α-carotene, β-carotene, and β-cryptoxanthin [4, 6]. β-Carotene is considered a major dietary source of vitamin A because it displays higher vitamin A activity than other carotenoids, as upon cleavage it yields 2 molecules of retinal [7, 8]. The recommended β-carotene intake level is around 2-4 mg/day [6, 7, 9], though no dietary reference intake (DRI) such as a recommended dietary allowance (RDA) exists. Similarly, the traditional conversion factor for β-carotene (6 mg) to retinol (1 mg) was observed to be 2-folds greater than that of other carotenoids (12 mg). As per the German National Nutrition Survey I and II, a significant proportion (25%–48%) of retinol equivalent intake comes from β-carotene, hence as based on these 2 surveys, the recommended β-carotene intake from the diet is around 2–4.5 mg/day [6, 7, 9].
Unfortunately, bioavailability of most carotenoids, including that of β-carotene, is low. This low bioavailability of β-carotene from natural food sources has mainly been attributed to the low release from food items, as it is present bound to proteins, and its release relies on cell wall degradation, in combination with a high apolarity and low efficacy of micellarization [10–12], a prerequisite for its cellular uptake [13, 14]. β-Carotene can be obtained from dark leafy green vegetables, oranges and yellow vegetables and fruits [7, 14, 15]. Its low bioavailability from natural sources renders the extraction and isolation of β-carotene important for the production of either supplements or fortified food matrices with enhanced bioavailability to support maximum health benefits.
Specific population groups, such as neonates and elderly persons, as well as patients suffering from gastrointestinal diseases and impaired digestion are compelled to acquire vitamin A from vitamin A rich diets, fortified foods and pharmaceutical supplements to meet their daily requirements [16]. The mechanism of β-carotene uptake and the factors thought to affect its absorption have been highlighted previously [17–21]. Absorption of β-carotene is a sequential process, it needs to be micellarized due to its low water solubility and then taken up by cells, followed by its transport to the golgi system of cells and secretion via chylomicrons, and finally it needs to be redistributed into lipoproteins by the liver [20, 22].
Bioaccessibility and Bioavailability
To exert its biological effects, β-carotene needs first to be released from food matrix and become bioaccessible. The bioaccessibility can be defined as the proportion or quantity which is released from the food matrix in the GI tract and becomes available for absorption by the enterocytes.
where Br: Amount of β-carotene release in GIT fluid as a result of digestion of food matrix, Bt: Total amount of β-carotene present in the food matrix, Be: β-Carotene content in the bile and pancreatic solutions secreted into the duodenal compartment.
Bioavailability can be defined as the fraction of administered β-carotene (the total β-carotene contained in a consumed food item) that eventually reaches the systemic circulation, though one may also include distribution, metabolism and excretion into the equation (ADME). The major factors limiting the bioavailability of β-carotene should be discussed as this information may facilitate the design of foods that can effectively deliver β-carotene. The currently available information on how β-carotene is released from food matrices and absorbed by the GIT is presented in Figure 1. For any fat-soluble compound present in food, the bioavailability (F) can be defined as follows [23],
where FB: Bioaceessibility coefficient of the fat-soluble compound released in the gastro-intestinal (GIT) fluid, FT: Transportation coefficient of the released fat-soluble compound that is transported across the GIT epithelium, FM: The fraction of the fat-soluble compound that ultimately arrives in the systemic circulation remaining after metabolism.
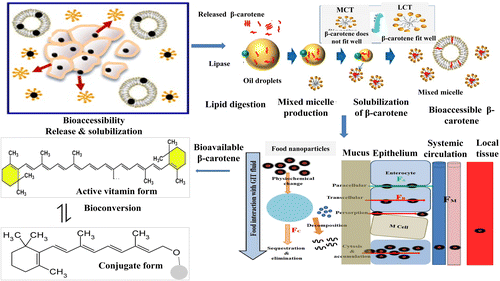
Carotenoid digestion and cellular uptake
The fate of β-carotene in the GIT is controlled by factors that are intimately associated with digestive conditions, especially fat digestion [4, 24–28]. These factors include release from the matrix, dissolution into lipid droplets, emulsification, incorporation into mixed micelles, diffusion across the unstirred water layer and admission into enterocytes via the cell membrane of the small intestine [29]. The processing of β-carotene in the GIT follows a multistage process, involving enzymatic and physiochemical reactions in the stomach and the small intestine (Figure 2).
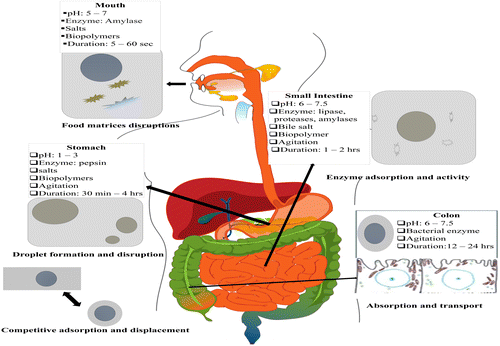
The (low) pH of the gastric digestive fluid also affects the absorption of β-carotene by minimizing the surface charge (zeta potential) of micellarized β-carotene and allowing their adherence to the cells, hence enhancing its bioavailability [30, 31]. Very few data are available on the effect of the pH of GIT fluids on β-carotene. Especially in protein-rich food items such as some legumes, protein-catalyzing enzymes, including trypsin and pepsin (weak effect), can be assumed to drive β-carotene absorption by digesting the proteins present in plastids that bind β-carotene [32, 33] and limit its release from plant sources [30, 34–36]. Additionally, in the small intestine, duodenal digestive enzymes (amylases, lipase and protease) also enhance the bioavailability by releasing β-carotene from food matrices [36, 37]. Furthermore, there is uncertainty whether carotenoids can be absorbed in the colon. It is well reported that a significant proportion of carotenoids is potentially bioavailable in the colon, as merely 5–50% are absorbed in the small intestine [21]. However, a major proportion (50–90%) of carotenoids reacts to unknown compounds and merely 10–50% of the carotenoids remain intact after colonic fermentation [21, 34, 35].
Mechanism of absorption
The absorption of β-carotene is believed to be taking place in part by a non-saturable passive diffusion process [19]. In addition, recent reports on the human intestinal Caco-2 cell line (Caucasian colon adenocarcinoma) [22, 38, 39] and knock-out animal studies [40, 41] verified the involvement of enterocyte cell membrane proteins participating in the uptake of β-carotene on the brush-border side of the enterocytes. Absorbed fat-soluble compounds (tocopherol and vitamins A and D) have also been reported to be transported by such proteins such as SR-BI (scavenge receptor class B type 1) and CD 36 (cluster determinant 36) [19].
At low dietary concentration, β-carotene is thought to undergo protein-mediated transport, while passive diffusion appears to predominate at higher concentrations (pharmacological concentrations) [40, 42]. In living unanesthetized rats, β-carotene absorption followed linear passive diffusion when its administered concentration was in the range of 0.5 to 11 mM. However, the threshold concentration where the mode of β-carotene shifts its nature from protein-mediated transport to predominantly passive diffusion still needs to be determined. However, very little evidence related to this matter has been reported. Furthermore, it was also hypothesized that the bioavailability of β-carotene may be influenced by simultaneous ingestion of other carotenoids. For example, reduced bioavailability of lutein [43, 44] and canthaxanthin [45, 46] were witnessed in the presence of β-carotene. This could be due to competitive effects during micellization or cellular uptake, as well as interactions among carotenoids following cellular uptake [47], but data regarding β-carotene absorption in the presence of other carotenoids are limited.
Taken together, more systematic knowledge on β-carotene regarding its release from the food matrix, its mechanism of transfer from the oil phase to mixed micelles, and the effect of bile salts on β-carotene solubility, its localization in micelle, the role of digestive enzymes, and interactions with other fat-soluble compounds is needed. Such information is also critical for selecting appropriate digestion models and conditions for prediction β-carotene bioavailability. Figure 3 gives an overview of key factors influencing β-carotene bioavailability.
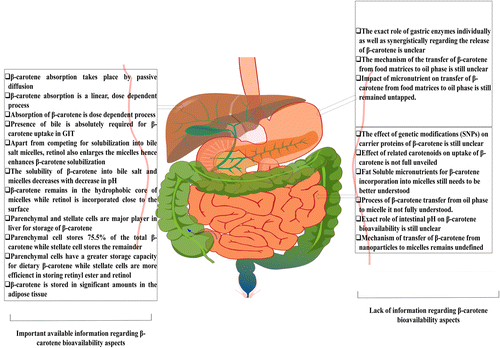
Possible variables influencing the bioavailability of β-carotene
To become available to enterocytes and to be absorbed in the GIT, β-carotene must be released from the food matricesin which it is physically or chemically bound. These aspects are reviewed in the following sections.
β-Carotene status in food
The absolute bioavailability of β-carotene is limited by the amount of β-carotene in the food [48–50]. In rats, β-carotene uptake and its bioconversion into vitamin A were found to be highly dependent on the β-carotene content in their diet [49], i.e. higher liver vitamin A concentrations were observed in those rats fed with a diet with higher β-carotene content. Furthermore, the studies on post-digestive recovery and efficiency of micellarization of β-carotene among 10 varieties of cassava fruit with a similar content of β-carotene clearly demonstrate the impact of genotypic variations on the release of β-carotene from the food matrix during in vitro digestion [48].
The low absorption efficiency of β-carotene from natural plant sources contributes much to vitamin A deficiencies. These deficiencies compel individuals to acquire vitamin A from sources beyond the regular diet to meet daily needs. In many countries with low meat intake, dietary vitamin A is mostly obtained in the form of β-carotene. Additional forms of vitamin A such as retinyl palmitate, retinyl acetate, and retinol can be obtained from dietary and pharmaceutical supplements or fortified foods.
β-Carotene absorption must be highly synchronized with vitamin A status in the host for optimal vitamin A homeostasis. In 1997, the standing committee of the US Department of Agriculture (USDA) established recommended dietary allowances (RDA values) of vitamins for specific age and gender groups to assure optimum intake but also to prevent vitamin overdosing [51]. Although accurate determination of daily vitamin A intake is challenging due to variations in food consumption patterns and differences in host metabolism, among other the USDA has released RDAs for different sets of populations such as 900 μg (adults and children ≥ 4 years), 500 μg (infants through 12 months), 300 μg (children 1 through 3 years) and 1,300 μg (pregnant women and lactating women) per day and capita. To prevent overdosing UL (upper tolerable intake levels) have also been defined.
Dietary pro-vitamin A carotenoids: α-carotene, β-carotene and β-cryptoxanthin
β-Cryptoxanthin, α-carotene, and β-carotene are significant contributors to dietary vitamin A intake. The molecular structures of these compounds are presented in Figure 4. The absorption of β-carotene is controlled by the intestinal homeobox ISX (protein transporters), and the rate of its absorption depends on its affinity (affinity of β-carotene with protein transporters) and concentration, which may be influenced by the presence of other molecular forms of vitamin A [43, 47].
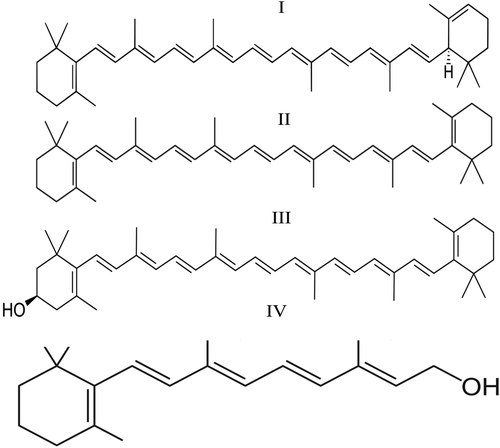
Bioavailability of various forms of β-carotene and dosing
β-Carotene cleavage by beta-carotene oxygenase 1 (BCO1) results in two molecules of active vitamin A compared to α-carotene or β-cryptoxanthin (yielding 1 molecule of retinal) during its bioconversion [7, 8]. In addition, cis-trans isomerism may influence the bioavailability of carotenoids. The result of a study comparing the bioavailability of the cis form of β-carotene with the trans form of β-carotene showed that the trans-form was 1.5-fold more bioavailable than of the cis-form [52]. This finding was supported by data produced in subsequent studies [47, 53–55]. These discrepancies in bioavailability and bioconversion between the carotenoids have been observed among individuals following ingestion of identical doses of carotenoids [56, 57]. The differences in bioavailability between α-carotene and β-carotene could be due to differences in the release rate of the two isomers from their matrix in which they were contained (α-carotene in capsule form while β-carotene in carrot) [56].
As mentioned, plasma β-carotene levels depend on the quantity of total β-carotene consumed. Generally, poorer fractional absorption efficiencies were observed following the ingestion of higher doses [58]. Prince and Frisoli demonstrated that the ingestion of β-carotene divided into 3 separate doses (with each meal) resulted in a higher β-carotene plasma level compared to that following a single high dose, but they were not able to determine the optimal dose [27]. Notably, after in vitro digestion, only 2.1% of the β-carotene was bioaccessible from red grapefruit in spite of having a high β-carotene concentration [59]. On the other extreme, administration of 45 mg of β-carotene per day resulted in yellowing of the skin (carotenodermia), while subjects consuming 15 mg β-carotene per day did not show such signs [60]. Based on the above observations, it can be suggested that ingestion of multiple small doses of β-carotene will result in higher bioavailability rather than the ingestion of high dose. However, more studies need to be conducted in order to draw more firm conclusions regarding the relation between dosing and fractional absorption.
Food matrix
The release from the food matrix is the first stage of β-carotene absorption, and this step starts with the physical degradation of the food matrix, i.e. mastication in the oral cavity. β-Carotene naturally exists in various forms within plant cells, i.e., in chloroplasts and chromoplasts. Furthermore, when β-carotene is present in chromoplasts, it can exist in different physical states, including solid crystals (carrot and tomato) or dissolved in oil droplets (pumpkin, mango and papaya) [13, 53, 61, 62]. This phenomenon could result in a more readily extraction of oil-dissolved β-carotene as compared to crystalline β-carotene during gastro-intestinal digestion.
The effects of the physical form of β-carotene on its bioavailability have indeed been studied. Schweiggert et al. [53] reported different bioavailability of β-carotene from various plant sources, such as 10.1% from mango, 5.3% from papaya, 3.1% from tomato and 0.5% from carrot [53]. These reports suggest the high bioavailability of β-carotene when it is present in dissolved form in oil droplets rather than insolid crystals form. The bioavailability of β-carotene also depends on the ripeness of the fruit and vegetable, associated with the softness of the cell walls [63].
β-Carotene release is largely dependent on the degree of structural disruption of the food matrix, which can be enhanced by applying various food processing techniques prior to ingestion [9, 57]. The improvement of β-carotene bioavailability by thermal processing is due to softening, disruption of plant cells and denaturation of protein-carotenoid complexes [13, 64, 66]. An in vivo study showed that β-carotene plasma levels could be increased three-fold after thermal processing of raw carrot and spinach (i.e., 121 °C for 40 min) after canning and sterilization [67]. These observations were further supported by the approximately 1.25-fold improved β-carotene plasma levels achieved when commercial carrot puree underwent retort processing in addition to cooking [64]. Furthermore, improvements in the bioavailability of β-carotene due to thermal processing have been observed in various studies [26, 28, 65, 66, 68]. Discrepancies between the bioavailability results from in vivo and in vitro studies (the bioavailability was higher in vivo) must be considered when extrapolating the in vitro derived bioaccessibility results to bioavailability of β-carotene in vivo for a given processed food.
Mechanical processing (pureeing) also enhances the release of β-carotene by reducing particle size, increasing the surface area available to digestive enzymes and thus improving the bioavailability of β-carotene. Improved bioavailability of β-carotene using food processing techniques such as crushing, cooking and blanching have been observed in various studies [5, 26, 69, 70]. An in vivo study evaluated the contributions of mechanical processing (21% increase as compared to raw food) and thermal processing (27% increase as compared to raw food) to improving bioavailability and observed that thermal processing was more effective for enhancing the bioavailability [26]. Contrary to this, no significant effect on the bioavailability of β-carotene under various pressure treatments was witnessed [71]. In contrast, in vitro bioaccessibility was considerably higher (approximately 2-folds) from food particles ≤125 μm than from particles larger than 126–160 μm [66]. The above observations indicated that higher bioavailability of β-carotene can be achieved by the simultaneous application of thermal as well as mechanical processing, but the exact conditions needs to be further evaluated. Additional studies on the simultaneous effects of thermal and mechanical processing on the bioavailability of β-carotene are necessary but promising.
Dietary lipids
Lipids are generally the most commonly known carriers for delivering β-carotene, and they are believed to be crucial players in delivering liposoluble micronutrients in general. Lipids control the absorption of fat-soluble micronutrients from food through a multistage process. The process begins with diffusion of lipophilic micronutrients from food matrices into the oil phase within which lipophilic micronutrients remain solubilized. In the following stage, these lipids stimulate the secretion of bile juice, which promotes mixed micelle formation. Thereafter, the enzymes in the GIT metabolize these lipids and cleave e.g. fatty acids and phospholipids, and these products have emulsifying properties and continuously form more micelles which can dissolve more lipophilic nutrients [4, 9, 72]. In the final stage, following cellular uptake, these lipids facilitate the release of lipophilic nutrients from the enterocytes in the form of chylomicron sequestration, preventing accumulation of β-carotene in the enterocytes and enhancing β-carotene absorption into the circulatory system. Therefore, these stages are influenced by the quantity and type (e.g. length) of the triglycerides and phospholipids, among other [72, 73].
Quantity of lipids (triglycerides)
A 2.5-fold increase in β-carotene plasma levels was observed after ingestion of β-carotene along with dietary fat (200 g) compared to the undetectable change in plasma levels when β-carotene was ingested in the absence of dietary fat [27]. Furthermore, increased β-carotene plasma levels were reported after ingestion of salad prepared with avocado (150 g avocado) as well as avocado oil (24 g) [74] compared to salad alone.
In contrast, with increase in dietary fat as well as fiber content from 10% to 30% in diet it was observed that vitamin A stores were higher, whereas hepatic β-carotene stores were lower. This phenomenon was attributed to the enhanced bioconversion of β-carotene into vitamin A with increased dietary fat ingestion [75]. However, an increase in β-carotene plasma level was observed when 8 mg of β-carotene was ingested with increasing amounts of fat (from 3 g to 36 g). These observations suggested that a minimum threshold of dietary fat is required to facilitate the absorption of β-carotene (3 g of dietary fat for 8 mg β-carotene). However, above that threshold (3 g fat for 8 mg β-carotene), additional dietary fat does not significantly influence the bioavailability of β-carotene. The amount of dietary fat required for optimal β-carotene bioavailability is still unclear [67, 76]. However, in one study the minimum amount of dietary fat required for optimum absorption of β-carotene was suggested to be 5 g of fat per meal [62]. This assumption was supported by a study where the addition of 20% of cooked oil to homogenized carrot pulp significantly increased the in vitro accessibility of β-carotene [26].
Type of fatty acids
The first report addressing the types of fatty acids was Borel’s study in which reduced β-carotene bioavailability was observed upon addition of fatty acids with certain chain length, i.e., β-carotene bioavailability was higher in the presence of long-chain fatty acids than that with medium-chain fatty acids [24]. These observations suggested that in addition to the quantity of fat, the type of triglycerides influences the bioavailability of β-carotene. More specifically, it is possible that some medium-chain fatty acids are absorbed via the portal vein and thus not result in increased chylomicron sequestration. Several studies have validated this hypothesis [28, 42, 77–79]. The increase in the bioavailability of β-carotene with increasing fatty acyl chain length may also be explained by the influence of chain length (8–18 carbon chain) which favors mixed micelle formation. Furthermore, the efficiency of micelle formation was found to increase from 4.9% to 8.6% to 14.9% as the acyl chain length of the fatty acids increased from 4 carbons to 8 carbons to 18 carbons, respectively [77]. Unlike long-chain fatty acids, medium-chain fatty acids (which do not easily integrate into micelles) may hamper β-carotene absorption by increasing the micellar size, decelerating the absorption of β-carotene by enterocytes [42]. Nevertheless, analogous results were not observed when pharmacological doses of β-carotene were administered with medium-chain triglycerides. These observations could indicate a limited effect of lipid quantity and quality on β-carotene bioavailability [80].
Another factor may be the degree of saturation of a fatty acid. Ingestion of β-carotene along with monosaturated fatty acids and saturated fatty acids did not appear to significantly affect its bioavailability [77]. High bioavailability of β-carotene was recorded in the presence of unsaturated vegetable oil when compared to saturated vegetable oil, this phenomenon could be attributed to increased assembly (micelles) and secretion of chylomicrons [81, 82] as micelle derived from unsaturated fatty acids stimulate secretion of the lipophiles to a higher magnitude than micelles derived from the saturated fatty acids. But more scientific evidences are required to draw firm conclusion.
Furthermore, the bioaccessibility of β-carotene dissolved in digestible oil droplets was significantly higher than that from droplets derived from indigestible oil [83]. This phenomenon is attributed to the differences in stimulating mixed micelle formation as a result of digestion of digestible oil, which is higher than that from oil droplets derived from indigestible oil.
Dietary fiber
Dietary fiber is also believed to be a crucial factor influencing the fate of β-carotene in the GIT. It may influence the bioavailability of β-carotene in several ways: a) hindering micelle formation; b) influencing triacylglycerol lipolysis and emulsification of fat-soluble food compounds; c) modulating the release of lipophilic nutrients from the fat droplets (oil phase); d) increasing the viscosity of chyme, limiting the diffusion of lipophilic compounds from micelles to enterocytes.
With the growing awareness about its health benefits, dietary fibers are being fortified in food, changing the natural composition of food. High fiber intake was assumed to be the reason for reduced absorption of β-carotene in some human trials. The first study supporting this assumption demonstrated that pectin had a negative effect on β-carotene plasma levels when purified β-carotene was ingested with a meal [84]. In contrast, dietary fiber did not negatively influence carotenenoid absorption when β-carotene was served with liquefied spinach [62]. This phenomenon was explained by cell wall disruption, i.e., once cells were disrupted, the addition of fiber did not negatively affect carotene absorption.
However, the negative effect of fiber was supported by a study in which 33%, 42% and 43% decrease in absorption was observed when female volunteers ingested β-carotene along with alginate, pectin and guar, respectively [85]. This reduction in plasma β-carotene levels compared with those in control volunteers was attributed to two factors. On the one hand, soluble fiber increases fecal excretion of bile juices, limiting bile availability and mixed micelle formation [86, 87]. On the other hand, soluble dietary fiber increases the viscosity of chyme, hindering the contact between micelles and enterocytes [4, 84, 88].
Also vitamin A storage depended on the type of dietary fibers, which was explained by a higher degree of conversion of β-carotene into vitamin A when β-carotene was ingested with oat gum compared to citrus pectin [75]. In contrast to these observations, Unlu et al. [74] found that despite its high fiber content, ingestion of avocado fruit (150 g) improved the bioavailability of β-carotene, i.e. β-carotene plasma levels were found to be 7.9 ± 3.0 nmol/L after 9 h of test meal intake containing 150 g of avocado fruit as compared to 1.9 ± 0.5 nmol/L after 9 h of consuming a meal without avocado fruit. The effect was attributed to the higher intake of lipids, and thus the ratio of lipids to fiber should likely be taken into account. In summary, dietary fiber appears to hamper the absorption of β-carotene, with effects depending on the type and amount of fiber, and other compounds present within the specific test meals served. Further studies on the effects of fiber on carotenoid bioavailability are warranted.
Interaction with other micronutrients
Vitamins A, D, E, and K and related carotenoids (α-carotene, β-cryptoxanthin and xanthophylls) follow common β-carotene absorption patterns and may cause competition for β-carotene uptake in the GIT [89, 90]. This hypothesis was validated by a study on the Caco-2 cell line, which showed that other carotenoids hinder β-carotene absorption because polar carotenoids are more efficiently micellized and taken up in the GIT than the less polar β-carotene [73, 91, 92]. Furthermore, an in vitro study demonstrated that the rates of absorption of lutein and zeaxanthin exceeded those of β-carotene in Caco-2 intestinal cells [93].
The absence of epoxy-carotenoids, such as neoxanthin, violaxanthin, lutein epoxide and carotenol fatty acid esters in human plasma, despite their high concentrations in diets, could also be the result of such negative interactions, limiting the absorption of these molecules in the intestine, or alternatively, discriminations during their further metabolism [94].
However, the bioavailability of lutein was 5-fold higher than that of β-carotene, though their relative plasma responses may not reflect the true difference in their bioavailability levels [94]. This phenomenon can be explained also by bioconversion of absorbed β-carotene into vitamin A, as lutein does not undergo an analogous process [95]. The results of supplementation trials have suggested unfavorable interactions among carotenoids [43]. Unlike lycopene, lutein negatively influenced β-carotene absorption but did not affect β-carotene bioconversion into vitamin A when administered simultaneously [43].
Phytosterols (plant sterols), are widely used as functional ingredients to inhibit cholesterol uptake in the GIT. Because the absorption of β-carotene follows a path similar to that of other fat-soluble compounds, phytosterols are assumed to also impair β-carotene absorption. This hypothesis was validated by several reports in which the absorption of β-carotene was hindered by phytosterols [96–99].
Soluble proteins have also demonstrated inhibitory effects on the incorporation of β-carotene into micelles, possibly due to the prevention of enzyme access to the lipid droplets and transition into mixed micelles [30, 100]. However, proteins may also by themselves act as emulsifying agents and could also positively influence β-carotene bioaccessibility [101]. The surface charge on gastric micelles plays a key role in the absorption of β-carotene, and the charge is mainly due to phospholipids, fatty acids and proteins incorporated into the micelles. Phospholipids are likely a limiting factor as they drive micelle formation during digestion and hence may control the fate of β-carotene in the GIT. This hypothesis has been validated by several studies [102–106].
Enhancers and inhibitors of β-carotene absorption
The literature describes several agents that improve or hamper β-carotene uptake in the intestine. These agents can be naturally present in the food or may be supplemented to enhance the uptake of β-carotene.
Inhibitors of β-carotene uptake
Patients suffering from obesity also consume various anti-obesity drugs (for instance 60 mg three times/day Orlistat as per FDA) and fat replacers (e.g. olestra and other sucrose polyesters) to decrease their BMI and specifically body fat. On one hand, anti-obesity drugs such as Orlistat (a sucrose polyester) inhibit lipid digestion by hampering intestinal lipase while on the other hand fat replacers hamper the uptake of triglycerides. As β-carotene follows the same route as triglycerides in the intestine, these anti-obesity agents can hamper β-carotene bioavailability, thus reducing its uptake. For instance, a 31% decrease in β-carotene plasma levels (31%) was observed when 94 μg retinyl palmitate was co-ingested with one gram of sucrose polyester [107–109].
Enhancers of β-carotene uptake
In general, fat soluble bioactive compounds such as β-carotene and other carotenoids may be delivered, apart from the regular diet, through tablets, capsules or emulsions, liposomes or other delivery systems [110], and each of these systems may differ with respect to their release in the gastro-intestinal fluid and thus their extent of bio-accumulation in certain tissues.
β-Carotene delivery via specialized formulations (encapsulated in micro- or nanoparticles and micellar or liposomal formulations) may improve the bioavailability of β-carotene in the intestine [111]. The nano-scale of these delivery system offers several desirable functionalities (small particle size, high surface to volume ratio, encapsulation and protection and site-directed release), which not only protect β-carotene from hostile conditions of food as well as the GIT but also facilitate more micelle formation as a result of digestion of its components.
The ability of these formulations (with a particle size ≤0.45 μm) to enhance the absorption of β-carotene up to 70% was demonstrated in several studies [16, 39, 102, 104, 106, 112–116]. However, encapsulated β-carotene is also assumed to remain entrapped within undigested encapsulating nanomaterials rather than being released. These nanomaterials may release β-carotene near tight junctions with a diameter of 10Å, and may facilitate paracellular transport, hence bypassing organs that metabolize β-carotene. This transportation may lead to greater bioavailability of β-carotene from nanomaterials than that from supplements or fortified foods, but which may also differ in their bioactivity. These effects have not been well studied; therefore, conclusions about the impact of these nano-formulations on the bioavailability of β-carotene cannot be drawn, and this hypothesis must be validated by dedicated clinical trials.
Host related factors
The status of vitamin A and β-carotene in the host
The uptake of β-carotene is controlled by the β-carotene and vitamin A status of the host [117]. Nevertheless, determining the correlation between the host nutrient status and the uptake of β-carotene is very difficult. Due to its lipophilicity, β-carotene is deposited in adipose tissue and converted into vitamin A as required. Toxicity due to high β-carotene intake has not been reported so far [8], except for smokers [118].
Physiochemical interaction with GIT secretions
β-Carotene absorption peaks at specific values of ionic strength and pH, and changing these parameters beyond certain ranges can impact absorption. This hypothesis has been tested by several research groups. The absorption of β-carotene can be altered by changing the salt concentration during digestion. Progressive improvements in β-carotene uptake were observed when the bile concentration was increased [119].
High bile salt content may favor solubilization of monomeric β-carotene into micelles, thus improving its bioavailability. Relative to its crystal form, β-carotene incorporated into micelles has a greater ability to penetrate the membranes of enterocytes [120]. Changing the pH (0.3 to 8.3) of the perfusate in an in vivo study reduced the surface charge of both the micelles and the intestinal cell membrane, decreasing the repulsion between the micelles and the cell membrane and thus improving the uptake of β-carotene [42]. Furthermore, during fed conditions, pH and the β-carotene content in canola oil played crucial roles in micelle formation [87, 120]. Interestingly, Wright et al. [120] demonstrated a 28% increase in micelle formation under fasting conditions compared to that under non-fasting conditions. Wang et al. [87] observed that the low pH had a significant role in micelle formation during fasting but does not affect micelle formation during non-fasting conditions [84, 86, 87].
Age of the host
Variations in host physiological functions occur during aging. Age, which results in many physiological alterations, is thought to directly or indirectly influence β-carotene bioavailability [121, 122]. Age-derived changes in lipoprotein metabolism were alleged to decreased β-carotene uptake as well as postprandial transport [123]. This phenomenon was demonstrated in elderly women, and their GITs were less efficient at absorbing β-carotene than the GITs of younger women [124]. Declines in both pancreatic secretion and changes in the small intestine in elderly persons could also contribute to the low absorption. However, more focused experimental studies are necessary before drawing in-depth conclusions.
Gastric surgery/diseases
Initial reports indicated that patients with malfunctioning GITs, i.e., those with obstructive jaundice (low bile juice release) or pancreatic insufficiency as well as after gastric surgery, could exhibit reduced absorption of fat-soluble nutrients [125, 126]. In addition, low levels of β-carotene in plasma of patients suffering from small bowl syndrome and mal-absorptive syndrome also support this finding [127, 128].
Genetic variations
The uptake of β-carotene is controlled by several factors, such as the activity of enzymes associated with protein and fat digestion, bile secretion, and the activities of enzymes that convert β-carotene into vitamin A. Recently, Bohn et al. [21] comprehensively discussed these host-related factors which have significant effects on β-carotene absorption [21]. They have also highlighted effects of genetic variations on β-carotene absorption. Changes in the expression of genes coding for these proteins may cause complete or partial loss of their activity and thus decrease bioavailability. In addition, alterations in the genetic code of adjacent genes, i.e., promoter genes, may also affect the binding affinity of transcription factors, resulting in decreased activities of these protein transporters [129]. However, sufficient data on these factors have not been reported. Any genetic variation in genes coding for lipid-digestive enzymes and β-carotene-binding proteins may also result in changes in β-carotene absorption.
In vitro modeling of β-carotene digestion
Assessing bioavailability in human subjects would be ideal, but in vivo investigations remain impractical due to the associated costs, genetic variations among populations and the time required for such studies [130, 131], notwithstanding ethical restrictions. The application of in vitro digestion models is becoming more popular because of their ability to investigate several samples simultaneously, their rapidity, their superior reproducibility and their cost efficiency [132, 133]. Although in vitro digestion processes to determine bioavailability aspects of bioactive agents (such as β-carotene) have been developed and refined over time, and consensus models have been proposed [132], no standard digestion model exists for determining β-carotene bioavailability. The composition of the digesta and digestion conditions such as shaking speed [101] certainly influences bioaccessibility, and the in vitro digestion model should be reviewed to determine the key factors that must be considered during every stage of digestion [9, 120]. Despite of ability to investigate several samples simultaneously, in vitro digestion models also carry some limitations such as lacking simulation of peristalsis of the stomach, lack on control regarding oxygen concentration and lack a description of obtaining the bioaccessible fraction of released β-carotene [134].
Static vs. dynamic digestion system
Proper selection of an in vitro digestion model is the first step in determining the true bioavailability of a nutrient. Two main types of in vitro digestion models can be distinguished, namely, static and dynamic, which have been widely adopted to evaluate the bioavailability of bioactive compounds. On one hand, the static digestion models simulate the physicochemical changes that occur during the digestion process (pH, enzymes and salt concentration) without replicating the fluid flow, peristalsis and mixing that occurs during digestion [135], while on the other hand dynamic models aim to simulate the changes of enzyme, mineral and bile concentrations and pH that are occurring during digestion [135, 136]. In addition, dynamic models may better account for controlling enzyme concentration, pH and simulation of mechanical forces. Selection of the proper in vitro digestion models depends entirely on the nature of the sample to digest and the scope of the measurement, mainly the number of samples to be analyzed, which is far more limited in the dynamic models, and also require typically larger volumes of sample.
Differences between the models have been studied. For example, the dynamic in vitro digestion model indicated higher bioavailability of β-carotene (87.1%) in almond butter than that determined using the static model (51.0%) [137], which could be due to incomplete grinding/emulsification/mixing of the protein rich meal in the static in vitro model [138]. The observations in this study indicated that the dynamic in vitro digestion model is more appropriate, i.e. physiological, for solid or semisolid food matrices, while the static in vitro models are more appropriate for simpler samples, such as β-carotene emulsions.
Gastric digestion
Selection of the appropriate in vitro digestion model depends on the sample and the scope of measurements. Several models have been used to assess the bioavailability of β-carotene, some of which are presented in Table 1. Garrett’s in vitro digestion model has been the most widely adopted in vitro digestion model for determining β-carotene bioavailability [139]. While determining the bioaccessibility of β-carotene in homogenized baby food, they observed that gastric digestion does not significantly improve the bioaccessibility. Therefore, regardless of the inclusion or exclusion of gastric digestion, the gastric phase of in vitro digestion studies solely depends on the type of sample and the scope of the measurements. When in the gastric digestion step, appropriate concentrations of bile and pancreatin were omitted, the pH became the most critical parameter for evaluating β-carotene dissolved in oil [12]. For example, less than 8% and 4% of β-carotene was transported to the aqueous phase in the absence of pancreatin and bile, respectively. However, emulsions of β-carotene encapsulated in whey protein require the gastric phase [139] and processing by pepsin.
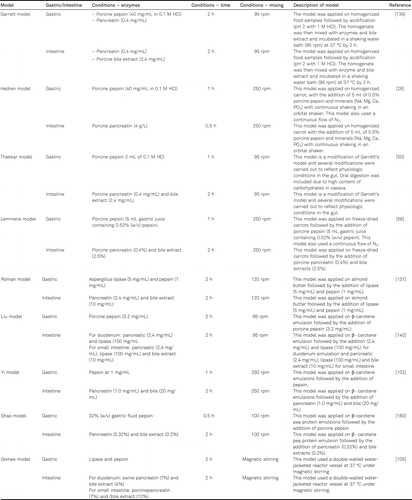
The pH must be carefully regulated because it may significantly influence the blood response, i.e., higher absorption of β-carotene was observed with increased acidity [123]. Similar observations have been reported previously [31]. It was concluded that a change in pH leads to an altered electrical potential of the oil–water interface (generally negatively charged) through which β-carotene penetrates the epithelium before being absorbed [31]. The addition of pepsin to catalyze protein degradation during the gastric phase was vital. Rich et al. [31] demonstrated that inclusion of pepsin resulted in a 40% increase in the transfer of β-carotene from raw carrot juice to olive oil. Pepsin played a crucial role in disrupting the structure of emulsions [140]. In addition to these factors, the importance of interfacial properties and micelle formation in the transfer of β-carotene from food matrices to oils must be emphasized. Pepsin should be included in the gastric digestion models when proteins are present, in order to simulate a more physiologically relevant digestion and to better estimate aspects of bioavailability.
Intestinal digestion
Several models have been adopted to assess aspects of bioavailability of β-carotene (Table 1). The digestion process was significantly affected by the concentration of bile as well as pancreatic enzymes during the determination of β-carotene bioaccessibility [101, 141]. In the absence of a standard model and intestinal enzyme concentrations, the bioaccessibility of a defined sample can significantly vary depending on the model used for evaluation. Despite β-carotene being consumed as part of the diet, several studies have simulated digestion in the fasting state [6, 120]. However, fed conditions are more physiologically relevant for investigating the bioaccessibililty of β-carotene, particularly from supplements and fortified products.
In vitro gastric digestion under fed conditions should imitate actual physiological conditions, i.e., processing of the sample in the oral phase and gastric phase, followed by pH regulation (pH 3–5 with hydrochloric acid to mimic gastric acid secretion during digestion) [130]. Duodenal conditions should involve a sufficient amount of pancreatin and bile, especially assuring sufficient lipase activity [130]. Wright et al. [120] used pancreatic and bile concentrations of 2.4 and 20.0 mg mL−1, respectively, to simulate fed conditions. No significant differences were observed when the bile concentration was increased (10.0 mg mL−1 and 20.0 mg mL−1) while keeping the pancreatin concentration constant (2.4 mg mL−1). These results indicate that a bile concentration of 10 mg mL−1 is sufficient to replicate fed conditions. In an in vivo study, the pH of the duodenum was found to decrease during digestion of a meal; therefore, pH values of 5 and 5.7 have been adopted to imitate fed duodenal digestive fluid [120, 142] as opposed to the otherwise frequently used pH of around 6 [120, 143].
Modeling β-carotene absorption
The uptake of β-carotene in the GIT may mainly occur through passive diffusion, and several in vitro digestion models can be used to simulate cellular uptake and absorption after digestion, using Caco-2 cell cultures as well as artificial membranes [4, 72, 144–146]. A parallel artificial membrane permeability assay, often employing a cellulose membrane of a specific molecule weight cut-off regarding kDa, can be used to simulate the intestine (particularly porosity) as it allows the diffusion of a bioactive agent from the digesta to a carrier solution [72]. Regarding cell-lines, currently, the Caco-2 cell line is the most common choice for determining β-carotene absorption in vitro, but this cell line is limited to static digestion models and is not suitable for the fluid flow regimes of the intestine [72]. Employing Caco-2 cell cultures with differentiated Caco-2 cells forming a monolayer not unlike the intestinal lining, methods include the addition of a dilute solution of the micelle phase after digestion to the Caco-2 cell culture, followed by measurement of the amount β-carotene taken up from the cells after a defined time period [139, 145, 147]. The accuracy of Caco-2 cells in absorption models has been validated by the correlation between bioavailability results obtained from in vivo and from in vitro studies [147]. Because the absorption of β-carotene is not fully understood and the factors that influence micelle formation may also influence absorption, and micellerization plays a vital role in β-carotene absorption, the inclusion of Caco-2 cells in the in vitro digestion model will provide a clearer understanding of β-carotene absorption [9]. This Caco-2 cellular model can be further refined, by e.g. growing the monolayer in transwells, thus studying transport of carotenoids and sequestration to the basolateral side [148]. In addition, co-culture cell models including also mucus-producing cell lines such as HT-29 MTX cells can be incorporated, and may better reflect the physiological conditions in the gut [149].
Comparison of in vitro digestion methods
Bile salt and pancreatin are used to imitate the fasting state of digestion process, which compromises bioaccessibility of β-carotene as compared to the fed state (with concentrations of 2.4 mg mL−1 pancreatin and 10 mg mL−1 bile salts) [6, 120]. Further, Roman et al. [137] developed an in vitro digestion protocol for high-lipid food matrices imitating the fed state of digestion by adding swine lipase A with enzymatic activity of 150 IU mg−1 to reproduce the effect of human gastric lipase. As these models are derived from Garrett et al. [150], the major difference was the use of glycodeoxycholate, taurocholate, and taurodeoxycholate as an alternative of the porcine bile salts mixture [105, 151–157]. Further, in gastric digestion models most of the models added acids to reproduce the release of HCl after ingestion of food [66, 137, 158]. Using an orbital shaking water bath at 250 rpm reproduced a higher bioaccessibility of β-carotene than that of a reciprocal shaking water bath at ~100 rpm [26], as this involves a much higher kinetic energy important for the emulsification process. However, according to Garrett and others model, most of the reports employed 95 rpm [101].
Summary and future research directions
The indispensible role of β-carotene in human health has been well discoursed in the literature. In order to demonstrate its in vivo effect in human, β-carotene needs to be released from food matrix and become bioaccessible in the GIT. In humans’ GIT, the bioaccessbility of β-carotene from plant sources was estimated to range from 5% to 65%. This variation in bioaccessibility of β-carotene has been attributed to those factors which critically govern its release from food matrix as well as facilitate it absorption in the GIT.
Here, we have reviewed those key factors which are regulating the bioacessiblility and also control its bioavailability. The plastid membrane, cell membrane and cell wall are main barriers in its release from food matrix. In addition, the complexity of food matrix, crystalline form, indigestibility of lipid, unsaturation in lipid, presence of dietary fibers, presence of other carotenoids, presence of antiobesity drugs, phytosterol, vitamin A status in host, age and malfunction of the GIT of the host were found to constitute major limiting factors in β-carotene bioaccessibility, while quantity of oil, length and unsaturation of the acyl-chain of triglycerides, micro-/nanoparticles, food processing and multiple dosings were found to be promoting factors for β-carotene bioaccessibility. The precise mechanisms governing β-carotene bioaccessibility and its bioavailability are still matter of debate and need more scientific evidences.
Furthermore, the selection of model systems is equally important in determining the bioaccessibility as well as the bioavailablity of β-carotene from given food matrices. A more systematic knowledge on β-carotene regarding its release from food matrix, mechanism of transfer from the oil phase to micelles, effect of bile salt on its solubility, its localization in micelle, role of digestive enzyme, and interaction with other fat soluble compounds would aid to a development of functional foods as well as pharmaceutical formations with improved functionalities.
In addition to above reviewed factors, there is lack of information of several critical factors which have possible roles in deciding the fate of β-carotene. The under-studied factors should receive more attention and investigation in the future. These are especially:
- •The pH of the gastric juice may affect the uptake of β-carotene. Little information is available on the vulnerability of isomeric forms of β-carotene as affected by pH variation in the GIT.
- •Digestive enzymes drive the release of β-carotene from food matrices but there is ambiguity in the exact role of these digestive enzymes on β-carotene bioavailability. The assessment of the impact of these digestive enzymes (lipase) individually or in combination and their concentration on β-carotene absorption will improve our understanding of their effect on β-carotene absorption. Simulation studies using rabbit lipase (as it shows similarity with human lipase) may aid to better understand lipid digestion in the stomach.
- •In the duodenum, digestive enzymes (amylases, protease and lipase) facilitated the release of β-carotene from food matrices. β-Carotene cleaved from food matrices during the digestion process is transferred from the oil phase (present in form of dietary fat) to the fat phase of chyme (micelles). However, there is ambiguity regarding the kinetics of β-carotene by which it is transferred from food matrices into micelles. More focused mechanistic studies need to be carried out to understand the transfer kinetics of β-carotene.
- •Micelles integrate all available lipophilic compounds present in the consumed meal within phospholipid bilayers. However, not many studies are available on additional fat soluble (other lipophilic vitamins, phytosterol etc.) compounds, and how they influence the transfer of β-carotene into micelles, and how the transport from the micelle into the enterocyte is happening.
- •Vitamins A, D, E can hamper the β-carotene uptake in the GIT but limited data is available at which concentrations they typically hamper β-carotene absorption in the GIT, possibly as this also depends on the food matrix.
- •β-Carotene remains emulsified in the lipid phase of food or embedded within particular proteins. Food items differ much in the complexity of their matrices. There are few studies addressing the impact of complexity of food matrices on β-carotene bioavailability. More comprehensive studies comparing the bioavailability of β-carotene are required in order to know the true effect of food matrix complexity on its bioavailability.
- •Effects of particular β-carotene formulations (micro/nanoparticles) on the absorption of β-carotene are not well understood.
- •Although β-carotene seems to follow passive diffusion within certain physiological concentrations, its poor solubility in the aqueous intra-cellular medium indicates the presence of cytosolic binding proteins in the enterocytes, but to date little work has been carried out on β-carotene binding proteins in the intestinal mucosa.
- •Related carotenoid forms follow a similar route but their affinity to transporters may differ due to a variation in molecular forms. Various membrane-bound transporter proteins are involved in the absorption of β-carotene which may result in discrepancies in the absorption of e.g. cis and trans forms of β-carotene. An investigation is needed to assess whether this bigotry is a result of physical properties between isomers of β-carotene or due to membrane bound transporters.
- •Finally, the bioconversion of β-carotene into vitamin A is one of the limiting factors in monitoring the fate of β-carotene following absorption.
Conclusions
The bioavailability of β-carotene is known to depend on food as well as host related factors. The variation in the bioavailability of β-carotene could be due to a multitude of individual factors such as its status in food matrix (quantity, location in plant cells, physical state in food), complexity of the food matrix (quantity and quality of food components such as dietary fiber, dietary lipids and other components), interaction with other micronutrients during absorption, degree of food processing, size of food particles, and presence of absorption inhibitors (fat replacers and anti-obesity drugs) or enhancers (nano-/micro-formulations). However, host related factors such as physiochemical interactions during GIT secretion (enzyme, salts and time of exposure) and other host related factors such as surgery, age, disease, obesity, genetic variations do play a role. Furthermore, selecting an appropriate digestion model is crucial for assessing bioaccessibility which should resemble the in vivo situation. Until now, Garrett’s in vitro digestion protocol was found to represent the most widely adopted model for studying β-carotene digestion in particular samples, though the recently re-defined Infogest model [159] has also found broad application. After a comprehensive review we have found that there are several factors that remain unaddressed in the present literature as emphasized above. In order to aid generating more in-depth knowledge about the impact of these factors on the bioavailability of β-carotene, more focused investigations in vitro and in vivo are needed, including those with labeled meals as is common in mineral research, such as with radio-labeled or stable-isotope labeled β-carotene.
References
1 . Micronutrient deficiencies. Vitamin A deficiency. Available from: https://www.who.int/nutrition/topics/vad/en/
2 . The vicious cycle of vitamin A deficiency: A review. Crit Rev Food Sci Nutr. 2017;57(17):3703–14. https://doi.org/10.1080/10408398.2016.1160362.
3 .
Carotenoids: A brief overview on its structure, biosynthesis, synthesis, and applications . In: Zepka LQ, editor. Progress Carotenoid Research. IntechOpen; 2018. p. 1–15. https://doi.org/10.5772/intechopen.795424 . Soluble fibers inhibit carotenoid micellization in vitro and uptake by Caco-2 cells. Biosc Biotechnol Biochem. 2009;73(1):196–9. https://doi.org/10.1271/bbb.80510.
5 . Intestinal absorption and tissue distribution of carotenoids. J Nutr Biochem. 1997;8(7):364–77. https://doi.org/10.1016/s0955-2863(97)00060-0.
6 β-Carotene is an important vitamin A source for humans. J Nutr. 2010;140(12):2268S–85S. https://doi.org/10.3945/jn.109.119024.
7 . The challenge to reach nutritional adequacy for vitamin A: β-carotene bioavailability and conversion – evidence in humans. Am J Clin Nutr. 2012;96(5):1193S–203S. https://doi.org/10.3945/ajcn.112.034850.
8 . The contribution of β-carotene to vitamin A supply of humans. Mol Nutr Food Res. 2012;56(2):251–8. https://doi.org/10.1002/mnfr.201100230.
9 . Carotenoid bioavailability and bioconversion. Ann Rev Nutr. 2002;22(1):483–504. https://doi.org/10.1146/annurev.nutr.22.010402.102834.
10 . Lipid digestion, micelle formation and carotenoid bioaccessibility kinetics: Influence of emulsion droplet size. Food Chem. 2017;229:653–62. https://doi.org/10.1016/j.foodchem.2017.02.146.
11 . Comparative study on lipid digestion and carotenoid bioaccessibility of emulsions, nanoemulsions and vegetable-based in situ emulsions. Food Hydrocoll. 2019;87:119–28. https://doi.org/10.1016/j.foodhyd.2018.05.053.
12 . Designing emulsion droplets of foods and beverages to enhance delivery of lipophilic bioactive components – A review of recent advances. Int J Food Sci Technol. 2017;52(1):68–80. https://doi.org/10.1111/ijfs.13272.
13 . Absorption and transport of carotenoids. Ann New York Acad Sci. 1993;691(1):76–85. https://doi.org/10.1111/j.1749-6632.1993.tb26159.x.
14 . Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. British J Clin Pharmacol. 2013;75(3):588–602. https://doi.org/10.1111/j.1749-6632.1993.tb26159.x.
15 . Vitamin A deficiency. In: Semba RDBloem MW, editors. Nutrition and health in developing countries. 2nd ed. Totowa, NJ, USA: Humana Press; 2008. 377–433. https://doi.org/10.1007/978-1-59745-464-3_13.
16 . Public health programmes for vitamin A deficiency control. Comm Eye Health. 2013;26(84):69–70.
17 . Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. BBA - Mol Cell Biol L. 2012;1821(1):70–7. https://doi.org/10.1016/j.bbalip.2011.06.002.
18 . Mechanisms of digestion and absorption of dietary vitamin A. Ann Rev Nutr. 2005;25:87–103. https://doi.org/10.1146/annurev.nutr.25.050304.092614.
19 . Absorption of vitamin A and carotenoids by the enterocyte: Focus on transport proteins. Nutrients. 2013;5(9):3563–81. https://doi.org/10.3390/nu5093563.
20 β-Carotene in the human body: Metabolic bioactivation pathways–from digestion to tissue distribution and excretion. Proc Nutr Soc. 2019;78(1):68–87. https://doi.org/10.1017/s0029665118002641.
21 Host-related factors explaining inter-individual variability of carotenoid bioavailability and tissue concentrations in humans. Mol Nutr Food Res. 2017;61(6):1600685. https://doi.org/10.1002/mnfr.201600685.
22 CD36 and SR-BI are involved in cellular uptake of provitamin A carotenoids by Caco-2 and HEK cells, and some of their genetic variants are associated with plasma concentrations of these micronutrients in humans. J Nutr. 2013;143(4):448–56. https://doi.org/10.3945/jn.112.172734.
23 . Factors influencing the absorption of vitamin D in GIT: An overview. J Food Sci Technol. 2017;54(12):3753–65. https://doi.org/10.1007/s13197-017-2840-0.
24 Chylomicron β-carotene and retinylpalmitate responses are dramatically diminished when men ingest β-carotene with medium-chain rather than long-chain triglycerides. J Nutr. 1998;128(8):1361–7. https://doi.org/10.1093/jn/128.8.1361.
25 Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. J Clin Nutr. 2004;80(2):396–403. https://doi.org/10.1093/ajcn/80.2.396.
26 . Estimation of carotenoid accessibility from carrots determined by an in vitro digestion method. Eur J Clin Nutr. 2002;56(5):425. https://doi.org/10.1038/sj.ejcn.1601329.
27 . Beta-carotene accumulation in serum and skin. Am J Clin Nutr. 1993;57(2):175–81. https://doi.org/10.1093/ajcn/57.2.175.
28 . Content and in-vitro accessibility of pro-vitamin A carotenoids from Sri Lankan cooked non-leafy vegetables and their estimated contribution to vitamin A requirement. Food Sci Nutr. 2007;58(8):659–67. https://doi.org/10.1080/09637480701395580.
29 . Bioavailability of non-provitamin A carotenoids. Curr Nutr Food Sci. 2008;4(4):240–58. https://doi.org/10.2174/157340108786263685.
30 . Solubilization of carotenoids from carrot juice and spinach in lipid phases: I. Modeling the gastric lumen. Lipids. 2003;38(9):933–45. https://doi.org/10.1007/s11745-003-1147-0.
31 . Low pH enhances the transfer of carotene from carrot juice to olive oil. Lipids. 1998;33(10):985–92. https://doi.org/10.1007/s11745-998-0296-5.
32 Discovery of a chlorophyll binding protein complex involved in the early steps of photosystem II assembly in Synechocystis. The Plant Cell. 2014;26(3):1200–12. https://doi.org/10.1105/tpc.114.123919.
33 . Carotenoid biosynthesis in Arabidopsis: A colorful pathway. Am Soc Plant Biol. 2012;10:e0158. https://doi.org/10.1199/tab.0158.
34 . Determination of β-carotene and lutein available from green leafy vegetables by an in vitro digestion and colonic fermentation method. J Agric Food Chem. 2005;53(8):2936–40. https://doi.org/10.1021/jf0480142.
35 . Bioaccessibility of β-carotene, lutein, and lycopene from fruits and vegetables. J Agric Food Chem. 2006;54(15):5382–7. https://doi.org/10.1021/jf0609835.
36 . Methods for assessing aspects of carotenoid bioavailability. Curr Nutr Food Sci. 2010;6(1):44–69. https://doi.org/10.2174/157340110790909545.
37 . Rice bolus texture changes due to α-amylase. LWT - Food Sci. Technol. 2014;55(1):27–33. https://doi.org/10.1016/j.lwt.2013.09.014.
38 Release and absorption of carotenes from processed carrots (Daucus carota) using in vitro digestion coupled with a Caco-2 cell trans-well culture model. Food Res Int. 2011;44(4):868–74. https://doi.org/10.1016/j.foodres.2010.10.058.
39 . Formulation of oil-in-water β-carotene microemulsions: Effect of oil type and fatty acid chain length. Food Chem. 2015;174:270–8. https://doi.org/10.1016/j.foodchem.2014.11.056.
40 Class B scavenger receptor-mediated intestinal absorption of dietary β-carotene and cholesterol. Biochem. 2005;44(11):4517–25. https://doi.org/10.1021/bi0484320.
41 . A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila. Proc Nat Acad Sci. 2002;99(16):10581–6. https://doi.org/10.1073/pnas.162182899.
42 . Beta-carotene intestinal absorption: Bile, fatty acid, pH, and flow rate effects on transport. Am J Physiol Endocrinol Metabol. 1978;235(6):E686. https://doi.org/10.1152/ajpendo.1978.235.6.e686.
43 . Intestinal absorption, serum clearance, and interactions between lutein and beta-carotene when administered to human adults in separate or combined oral doses. Am J Clin Nutr. 1995;62(3):604–10. https://doi.org/10.1093/ajcn/62.3.604.
44 . Effect of simultaneous, single oral doses of beta-carotene with lutein or lycopene on the beta-carotene and retinyl ester responses in the triacylglycerol-rich lipoprotein fraction of men. Am J Clin Nutr. 1998;68(1):82–9. https://doi.org/10.1093/ajcn/68.1.82.
45 . Pharmacokinetics of beta-carotene and canthaxanthin after ingestion of individual and combined doses by human subjects. J Am College Nutr. 1994;13(6):665–71. https://doi.org/10.1080/07315724.1994.10718463.
46 . Interactions in the postprandial appearance of beta-carotene and canthaxanthin in plasma triacylglycerol-rich lipoproteins in humans. Am J Clin Nutr. 1997;66(5):1133–43. https://doi.org/10.1093/ajcn/66.5.1133.
47 . Carotenoid uptake and secretion by CaCo-2 cells β-carotene isomer selectivity and carotenoid interactions. J Lipid Res. 2002;43(7):1086–95. https://doi.org/10.1194/jlr.m200068-jlr200.
48 . In vitro screening of relative bioaccessibility of carotenoids from foods. Asia Pac J Clin Nutr. 2008;17(Suppl 1):200–3. https://doi.org/10.1007/s11130-014-0458-1.
49 . Kinetic characteristics of β-carotene uptake and depletion in rat tissue. J Nutr. 1984;114(10):1924–33. https://doi.org/10.1093/jn/114.10.1924.
50 . β-Carotene micellarization during in vitro digestion and uptake by Caco-2 cells is directly proportional to β-carotene content in different genotypes of cassava. J Nutr. 2007;137(10):2229–33. https://doi.org/10.1093/jn/137.10.2229.
51 . Dietary reference intakes: A risk assessment model for establishing upper intake levels for nutrients. Washington, DC, USA: National Academies Press; 1998. https://doi.org/10.17226/6432.
52 Discrimination in absorption or transport of beta-carotene isomers after oral supplementation with either all-trans-or 9-cis-beta-carotene. J Clin Nutr. 1995;61(6):1248–52. https://doi.org/10.1093/ajcn/61.6.1248.
53 . Influence of chromoplast morphology on carotenoid bioaccessibility of carrot, mango, papaya, and tomato. Food Chem. 2012;135(4):2736–42. https://doi.org/10.1016/j.foodchem.2012.07.035.
54 . The relative vitamin A value of 9-cis β-carotene is less and that of 13-cis β-carotene may be greater than the accepted 50% that of all-trans β-carotene in gerbils. J Nutr. 2002;132(9):2709–12. https://doi.org/10.1016/j.foodchem.2012.07.035.
55 . All-trans β-carotene preferentially accumulates in human chylomicrons and very low density lipoproteins compared with the 9-cis geometrical isomer. J Nutr. 1995;125(8):2128–33. https://doi.org/10.1093/jn/125.8.2128.
56 Plasma carotenoid response to chronic intake of selected foods and β-carotene supplements in men. Am J Clin Nutr. 1992;55(6):1120–5. https://doi.org/10.1093/ajcn/55.6.1120.
57 . Absorption, metabolism, and transport of carotenoids. FASEB J. 1996;10(5):542–51. https://doi.org/10.1096/fasebj.10.5.8621054.
58 . Bioavailability and bioconversion of carotenoids. Ann Rev Nutr. 1998;18(1):19–38. https://doi.org/10.1146/annurev.nutr.18.1.19.
59 . Xanthophyll carotenoids are more bioaccessible from fruits than dark green vegetables. Nutr Res. 2007;27(5):258–64. https://doi.org/10.1016/j.nutres.2007.04.002.
60 Bioavailability of beta-carotene in humans. Am J Clin Nutr. 1988;48(2):298–304. https://doi.org/10.1093/ajcn/48.2.298.
61 . Lack of improvement in vitamin A status with increased consumption of dark-green leafy vegetables. Lancet. 1995;346(8967):75–81. https://doi.org/10.1016/s0140-6736(95)92111-7.
62 . The food matrix of spinach is a limiting factor in determining the bioavailability of β-carotene and to a lesser extent of lutein in humans. J Nutr. 1999;129(2):349–55. https://doi.org/10.1093/jn/129.2.349.
63 . Impact of the stage of ripening and dietary fat on in vitro bioaccessibility of β-carotene in ‘Ataulfo’ mango. J Agric Food Chem. 2008;56(4):1511–6. https://doi.org/10.1021/jf072751r.
64 . α-and β-Carotene from a commercial carrot puree are more bioavailable to humans than from boiled-mashed carrots, as determined using an extrinsic stable isotope reference method. J Nutr. 2002;132(2):159–67. https://doi.org/10.1093/jn/132.2.159.
65 . Bioaccessibility of carotenes from carrots: Effect of cooking and addition of oil. Innov Food Sci Emerg Technol. 2007;8(3):407–12. https://doi.org/10.1016/j.ifset.2007.03.014.
66 . Particle size reduction leading to cell wall rupture is more important for the β-carotene bioaccessibility of raw compared to thermally processed carrots. J Agric Food Chem. 2010;58(24):12769–76. https://doi.org/10.1021/jf102554h.
67 . Bioavailability of β-carotene is lower in raw than in processed carrots and spinach in women. J Nutr. 1998;128(5):913–6. https://doi.org/10.1093/jn/128.5.913.
68 Carotenoid bioavailability in humans from tomatoes processed in different ways determined from the carotenoid response in the triglyceride-rich lipoprotein fraction of plasma after a single consumption and in plasma after four days of consumption. J Nutr. 2000;130(5):1189–96. https://doi.org/10.1093/jn/130.5.1189.
69 . Lycopene is more bioavailable from tomato paste than from fresh tomatoes. Am J Clin Nutr. 1997;66(1):116–22. https://doi.org/10.1093/ajcn/66.1.116.
70 . Serum β-carotene response to supplementation with raw carrots, carrot juice or purified β-carotene in healthy non-smoking women. Nutr Res. 1996;16(4):565–75. https://doi.org/10.1016/0271-5317(96)00035-8.
71 . Effects of high pressure processing on antioxidant activity, and total carotenoid content and availability, in vegetables. Food Sci Emerg Technol. 2007;8(4):543–8. https://doi.org/10.1016/j.ifset.2007.04.005.
72 . Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Res Int. 2012;46(2):438–50. https://doi.org/10.1016/j.ifset.2007.04.005.
73 Processing of vegetable-borne carotenoids in the human stomach and duodenum. Am J Physiol Gastrointest Liver Physiol. 2003;284(6):G913–23. https://doi.org/10.1152/ajpgi.00410.2002.
74 . Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J Nutr. 2005;135(3):431–6. https://doi.org/10.1093/jn/135.3.431.
75 . Amount of dietary fat and type of soluble fiber independently modulate post absorptive conversion of β-carotene to vitamin A in Mongolian gerbils. J Nutr. 2000;130(11):2789–96. https://doi.org/10.1093/jn/130.11.2789.
76 . Amount of fat in the diet affects bioavailability of lutein esters but not of α-carotene, β-carotene, and vitamin E in humans. Am J Clin Nutr. 2000;71(5):1187–93. https://doi.org/10.1093/ajcn/71.5.1187.
77 . Impact of fatty acyl composition and quantity of triglycerides on bioaccessibility of dietary carotenoids. J Agric Food Chem. 2007;55(22):8950–7. https://doi.org/10.1021/jf071687a.
78 Dietary fish oil–induced changes in the distribution of α-tocopherol, retinol, and β-carotene in plasma, red blood cells, and platelets: Modulation by vitamin E. Am J Clin Nutr. 1993;58(1):98–102. https://doi.org/10.1093/ajcn/58.1.98.
79 . Influence of dietary fat on β-carotene absorption and bioconversion into vitamin A. Nutr Rev. 2002;60(4):104–10. https://doi.org/10.1301/00296640260085831.
80 . Challenges to understanding and measuring carotenoid bioavailability. BBA Mol Basis Disease. 2005;1740(2):95–100. https://doi.org/10.1016/j.bbadis.2004.11.012.
81 . Unsaturated fatty acids promote bioaccessibility and basolateral secretion of carotenoids and α-tocopherol by Caco-2 cells. Food Funct. 2014;5(6):1101–12. https://doi.org/10.1039/c3fo60599j.
82 . Unsaturated fatty acids promote carotenoid bioavailability in vitro [poster presentation]. 2009.
83 . Nutraceutical nanoemulsions: Influence of carrier oil composition (digestible versus indigestible oil) on β-carotene bioavailability. J Sci Food Agric. 2013;93(13):3175–83. https://doi.org/10.1002/jsfa.6215.
84 . Plasma β-carotene response in humans after meals supplemented with dietary pectin. Am J Clin Nutr. 1992;55(1):96–9. https://doi.org/10.1093/ajcn/55.1.96.
85 . Some dietary fibers reduce the absorption of carotenoids in women. J. Nutr. 1999;129(12):2170–6.
86 . Binding of bile acids by dietary fiber. Am J Clin Nutr. 1978;31(10):S175–9. https://doi.org/10.1093/ajcn/31.10.s175.
87 . Preliminary study into the factors modulating β-carotene micelle formation in dispersions using an in vitro digestion model. Food Hydrocoll. 2012;26(2):427–33. https://doi.org/10.1016/j.foodhyd.2010.11.018.
88 . The potential for the improvement of carotenoid levels in foods and the likely systemic effects. J Sci Food Agric. 2000;80(7):880–912. https://doi.org/10.1002/(sici)1097-0010(20000515)80:7<880::aid-jsfa646>3.0.co;2-1.
89 . Influence of dietary fats and vitamin E on plasma and hepatic vitamin A and β-carotene levels in rats fed excess β-carotene. Nutr Cancer. 1990;14:111–6. https://doi.org/10.1080/01635589009514084.
90 . Vitamin E enhances the lymphatic transport of β-carotene and its conversion to vitamin A in the ferret. Gastroenterology. 1995;108(3):719–26. https://doi.org/10.1016/0016-5085(95)90444-1.
91 Carotenoids in biological emulsions: Solubility, surface-to-core distribution, and release from lipid droplets. J Lipid Res. 1996;37(2):250–61. https://doi.org/10.1016/0005-2760(95)00173-5.
92 . Main factors governing the transfer of carotenoids from emulsion lipid droplets to micelles. BBA Mol Cell Biol Lipid. 2001;1533(3):285–92. https://doi.org/10.1016/s1388-1981(01)00163-9.
93 . Assessment of lutein bioavailability from meals and a supplement using simulated digestion and Caco-2 human intestinal cells. J Nutr. 2004;134(9):2280–6. https://doi.org/10.1093/jn/134.9.2280.
94 . Separation and identification of carotenoids and their oxidation products in the extracts of human plasma. Anal Chem. 1992;64(18):2111–22. https://doi.org/10.1021/ac00042a016.
95 Bioavailability of lutein from vegetables is 5 times higher than that of β-carotene. Am J Clin Nutr. 1999;70(2):261–8. https://doi.org/10.1093/ajcn.70.2.261.
96 . High dietary intake of phytosterol esters decreases carotenoids and increases plasma plant sterol levels with no additional cholesterol lowering. J Lipid Res. 2004;45(8):1493–9. https://doi.org/10.1194/jlr.m400074-jlr200.
97 . Retinol, vitamin D, carotenes and α-tocopherol in serum of a moderately hypercholesterolemic population consuming sitostanol ester margarine. Atherosclerosis. 1999;145(2):279–85. https://doi.org/10.1016/s0021-9150(99)00078-7.
98 Both free and esterified plant sterols reduce cholesterol absorption and the bioavailability of β-carotene and α-tocopherol in normocholesterolemic humans. Am J Clin Nutr. 2004;80(1):171–7. https://doi.org/10.1093/ajcn/80.1.171.
99 . Diet supplementation with beta-carotene improves the serum lipid profile in rats fed a cholesterol-enriched diet. J Physiol Biochem. 2013;69(4):811–20. https://doi.org/10.1007/s13105-013-0257-4.
100 . Effects of dietary protein, fat and beta-carotene levels on beta-carotene absorption in rats. Int J Vitam Nutr Res. 2005;75(4):274–80. https://doi.org/10.1024/0300-9831.75.4.274.
101 Whey protein isolate modulates beta-carotene bioaccessibility depending on gastro-intestinal digestion conditions. Food Chem. 2019;291:157–66. https://doi.org/10.1016/j.foodchem.2019.04.003.
102 . The physicochemical stability and in vitro bioaccessibility of beta-carotene in oil-in-water sodium caseinate emulsions. Food Hydrocoll. 2014;35:19–27. https://doi.org/10.1016/j.foodhyd.2013.07.025.
103 . Effects of egg consumption on carotenoid absorption from co-consumed, raw vegetables. Am J Clin Nutr. 2015;102(1):75–83. https://doi.org/10.3945/ajcn.115.111062.
104 . Investigation into the in vitro release properties of β-carotene in emulsions stabilized by different emulsifiers. LWT Food Sci Technol. 2014;59(2):867–73. https://doi.org/10.1016/j.lwt.2014.07.045.
105 Physico-chemical stability and in vitro digestibility of beta-carotene-loaded lipid nanoparticles of cupuacu butter (Theobroma grandiflorum) produced by the phase inversion temperature (PIT) method. J Food Eng. 2017;192:93–102. https://doi.org/10.1016/j.jfoodeng.2016.08.001.
106 . Beta-carotene: Digestion, microencapsulation, and in vitro bioavailability. Food Bioprocess Technol. 2014;7(2):338–54. https://doi.org/10.1007/s11947-013-1244-z.
107 Decreased carotenoid concentrations due to dietary sucrose polyesters do not affect possible markers of disease risk in humans. J Nutr. 2003;133(3):720–6. https://doi.org/10.1093/jn/133.3.720
108 Olestra affects serum concentrations of α-tocopherol and carotenoids but not vitamin D or vitamin K status in free-living subjects. J Nutr. 1997;127(8):1636S–45S. https://doi.org/10.1093/jn/127.8.1636s.
109 . Factors influencing the uptake and absorption of carotenoids. Proc Soc Exp Biol Med. 1998;218(2):106–8. https://doi.org/10.3181/00379727-218-44275.
110 . Enhancing bio-availability of vitamin D by nano-engineered based delivery systems-An overview. Int J Curr Microbiol App Sci. 2017;6(7):340–53. https://doi.org/10.20546/ijcmas.2017.607.040.
111 , Improving bioavailability of vitamin A in food by encapsulation: An update. In: Daima HKPN NRanjan SDasgupta NLichtfouse E, editors. Nanoscience in Medicine. Vol. I. Cham, Switzerland: Springer; 2020. p. 117–45.
112 . Characterization and in vitro bioavailability of β-carotene: Effects of microencapsulation method and food matrix. LWT Food Sci Technol. 2014;57(1):42–8. https://doi.org/10.1016/j.lwt.2013.12.037.
113 . Physicochemical stability and in vitro bioaccessibility of β-carotene nanoemulsions stabilized with whey protein-dextran conjugates. Food Hydrocoll. 2017;63:256–64. https://doi.org/10.1016/j.foodhyd.2016.09.008.
114 . Control of β-carotene bioaccessibility using starch-based filled hydrogels. Food Chem. 2015;173:454–61. https://doi.org/10.1016/j.foodchem.2014.10.053.
115 . In vitro bioaccessibility and stability of beta-carotene in ethyl cellulose oleogels [Thesis]. Guelph, Ontario, Canada: The University of Guelp; 2016. https://atrium.lib.uoguelph.ca/xmlui/handle/10214/9716.
116 . Coating oil droplets with rice proteins to control the release rate of encapsulated beta-carotene during in vitro digestion. RSC Adv. 2016;6(77):73627–35. https://doi.org/10.1039/c6ra14943j.
117 ISX is a retinoic acid-sensitive gatekeeper that controls intestinal β, β-carotene absorption and vitamin A production. FASEB J. 2010;24(6):1656–66. https://doi.org/10.1039/c6ra14943j 10.1096/fj.09-150995.
118 The β-carotene and retinol efficacy trial (CARET) for chemoprevention of lung cancer in high risk populations: Smokers and asbestos-exposed workers. Cancer Res. 1994;54(7):2038s–43s. https://doi.org/10.1016/0169-5002(94)92177-6.
119 . Vitamin D-3 intestinal absorption in vivo: Influence of fatty acids, bile salts, and perfusate pH on absorption. Gut. 1978;19(4):267–72. https://doi.org/10.1136/gut.19.4.267.
120 . Influence of simulated upper intestinal parameters on the efficiency of beta carotene micellarisation using an in vitro model of digestion. Food Chem. 2008;107(3):1253–60. https://doi.org/10.1016/j.foodchem.2007.09.063.
121 . Steady-state serum concentration of alpha tocopherol not altered by supplementation with oral beta carotene. J Nat Cancer Inst. 1994;86(2):117–20. https://doi.org/10.1093/jnci/86.2.117.
122 . Factors in aging that effect the bioavailability of nutrients. Am J Clin Nutr. 2001;131(4):1359S–61S. https://doi.org/10.1093/jn/131.4.1359s.
123 . Gastric acidity influences the blood response to a beta-carotene dose in humans. Am J Clin Nutr. 1996;64(4):622–6. https://doi.org/10.1093/ajcn/64.4.622.
124 . Intestinal cholecalciferol absorption in the elderly and in younger adults. Clin Sci Mol Med. 1978;55:2013–20. https://doi.org/10.1042/cs0550213.
125 Vitamin D absorption: Consequences of gastric bypass surgery. Eur J Endocrin. 2011;164(5):827–32. https://doi.org/10.1530/eje-10-1126.
126 Use of a novel vitamin D bioavailability test demonstrates that vitamin D absorption is decreased in patients with quiescent Crohn’s disease. Inflamm Bowel Dis. 2011;17(10):2116–21. https://doi.org/10.1002/ibd.21595.
127 Prospective analysis of serum carotenoids, vitamin A, and tocopherols in adults with short bowel syndrome undergoing intestinal rehabilitation. Nutrition. 2009;25(4):400–7. https://doi.org/10.1016/j.nut.2008.10.003.
128 . Blood carotene in steatorrhea and the malabsorptive syndromes. Am J Med. 1957;22(3):373–80. https://doi.org/10.1016/0002-9343(57)90093-1.
129 . Polymorphisms in gene regulatory regions and their role in the physiopathology of complex disease in the post-genomic era. Saludpublica de Mexico. 2009;51:S455–62. https://doi.org/10.1590/s0036-36342009000900011.
130 . A physiological approach for preparing and conducting intestinal bioavailability studies using experimental systems. Food Chem. 2002;76(2):225–30. https://doi.org/10.1016/s0308-8146(01)00291-6.
131 . In vitro human digestion models for food applications. Food Chem. 2011;125(1):1–12. https://doi.org/10.1016/j.foodchem.2010.08.036.
132 INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat Protoc. 2019;14(4):991–1014. https://doi.org/10.1038/s41596-018-0119-1.
133 A standardised static in vitro digestion method suitable for food–An international consensus. Food Func. 2014;5(6):1113–24. https://doi.org/10.1039/c3fo60702j.
134 . Effects of legume processing on calcium, iron and zinc contents and dialysabilities. J Sci Food Agric. 2001;81(12):1180–5. https://doi.org/10.1002/jsfa.927.
135 . Relevance and challenges in modeling human gastric and small intestinal digestion. Trends Biotechnol. 2012;30(11):591–600. https://doi.org/10.1016/j.tibtech.2012.08.001.
136 . A human gastric simulator (HGS) to study food digestion in human stomach. J Food Sci. 2010;75(9):E627–35. https://doi.org/10.1111/j.1750-3841.2010.01856.x.
137 . Release and bioaccessibility of β-carotene from fortified almond butter during in vitro digestion. J Agric. Food Chem. 2012;60(38):9659–66. https://doi.org/10.1021/jf302843w.
138 Comparison of a static and a dynamic in vitro model to estimate the bioaccessibility of As, Cd, Pb and Hg from food reference materials Fucus sp. (IAEA-140/TM) and Lobster hepatopancreas (TORT-2). Sci Total Environ. 2011;409(3):604–11. https://doi.org/10.1016/j.scitotenv.2010.10.021.
139 . Development of an in vitro digestion method to assess carotenoid bioavailability from meals. J Agric Food Chem. 1999;47(10):4301–9. https://doi.org/10.1021/jf9903298.
140 . Investigation into the bioaccessibility and microstructure changes of β-carotene emulsions during in vitro digestion. Food Sci Emerg Technol. 2012;15:86–95. https://doi.org/10.1016/j.ifset.2012.04.002.
141 . Dietary and host-related factors influencing carotenoid bioaccessibility from spinach (Spinacia oleracea). Food Chem. 2011;125(4):1328–34. https://doi.org/10.1016/j.foodchem.2010.09.110.
142 Postprandial chylomicron and plasma vitamin E responses in healthy older subjects compared with younger ones. Eur J Clin Invest. 1997;27(10):812–21. https://doi.org/10.1046/j.1365-2362.1997.1960744.x.
143 . Determination of bioaccessibility of β-carotene in vegetables by in vitro methods. Mol Nutr Food Res. 2006;50(11):1047–52. https://doi.org/10.1002/mnfr.200600076.
144 . Digestive stability, micellarization, and uptake of β-carotene isomers by Caco-2 human intestinal cells. J Agric Food Chem. 2006;54(7):2780–5. https://doi.org/10.1021/jf0530603.
145 . Assessment of carotenoid bioavailability of whole foods using a Caco-2 cell culture model coupled with an in vitro digestion. J Agric Food Chem. 2004;52(13):4330–7. https://doi.org/10.1021/jf0530603.
146 . uptake, and transport of carotenoids from peppers (Capsicum spp.) using the coupled in vitro digestion and human intestinal Caco-2 cell model. J Agric Food Chem. 2010;58(9):5374–9. https://doi.org/10.1021/jf040028k.
147 . Bioaccessibility of carotenoids and vitamin E from their main dietary sources. J Agric Food Chem. 2006;54(23):8749–55. https://doi.org/10.1021/jf061818s.
148 . Comparison of the uptake and secretion of carotene and xanthophyll carotenoids by Caco-2 intestinal cells. Brit J Nutr. 2007;98(1):38–44. https://doi.org/10.1017/s000711450769446x.
149 . Inflammation related responses of intestinal cells to plum and cabbage digesta with differential carotenoid and polyphenol profiles following simulated gastrointestinal digestion. Mol Nutr Food Res. 2016;60(5):992–1005. https://doi.org/10.1002/mnfr.201500947.
150 . Accumulation and retention of micellar β-carotene and lutein by Caco-2 human intestinal cells. J Nutr Biochem. 1999;10(10):573–81. https://doi.org/10.1016/s0955-2863(99)00044-3.
151 . Characterization and antioxidant activity of β-carotene loaded chitosan-graft-poly (lactide) nanomicelles. Carbohydr Polymers. 2015;117:169–76. https://doi.org/10.1016/j.carbpol.2014.09.056.
152 . Bioavailability of β-carotene isomers from raw and cooked carrots using an in vitro digestion model coupled with a human intestinal Caco-2 cell model. Food Res Int. 2010;43(5):1449–54. https://doi.org/10.1016/j.foodres.2010.04.026.
153 . Carotenoid bioaccessibility from nine raw carotenoid-storing fruits and vegetables using an in vitro model. J Sci Food Agric. 2012;92(13):2603–10. https://doi.org/10.1002/jsfa.5768.
154 . Factors affecting the bioaccessibility of β-carotene in lipid-based microcapsules: Digestive conditions, the composition, structure and physical state of microcapsules. Food Hydrocoll. 2018;77:187–203. https://doi.org/10.1016/j.foodhyd.2017.09.034.
155 In vitro investigation of the bioaccessibility of carotenoids from raw, frozen and boiled red chili peppers (Capsicum annuum). Eur J Nutr. 2014;53(2):501–10. https://doi.org/10.1007/s00394-013-0555-1.
156 Modulation of chemical stability and in vitro bioaccessibility of beta-carotene loaded in kappa-carrageenan oil-in-gel emulsions. Food Chem. 2017;220:208–18. https://doi.org/10.1016/j.foodchem.2016.09.175.
157 . Encapsulation of β-carotene in alginate-based hydrogel beads: Impact on physicochemical stability and bioaccessibility. Food Hydrocoll. 2016;61:1–10. https://doi.org/10.1016/j.foodhyd.2016.04.036.
158 . Digestive stability of xanthophylls exceeds that of carotenes as studied in a dynamic in vitro gastrointestinal system. J Nutr. 2009;139(5):876–83. https://doi.org/10.3945/jn.108.103655.
159 . Gel-like pea protein Pickering emulsions at pH 3.0 as a potential intestine-targeted and sustained-release delivery system for β-carotene. Food Res Int. 2016;79:64–72. https://doi.org/10.1016/j.foodres.2015.11.025.


