Sensitivities of in vivo markers of arterial organ damage in patients with peripheral atherosclerosis
Abstract
Abstract.Background: Biomarkers of vascular diseases such as ankle-brachial index (ABI), peripheral pulse pressure (pPP), central pulse pressure (cPP), and pulse wave velocity (PWV) allow assessment of arterial organ damage (AOD). However, the utility of markers other than ABI in patients with peripheral arterial disease (PAD), which are also associated with a significant increase of cardiovascular events, remains unclear. Patients and methods: Asymptomatic (n = 21) and symptomatic patients (n = 46) with a positive sonography for PAD or history of lower limb revascularization were included. ABI, pPP, cPP, and PWV were assessed. PWV were performed using a brachial cuff-based method (aortic PWV (aPWV)) and oscillography (carotid-femoral pulse wave velocity (cfPWV)), respectively. The two methods for PWV were compared using Bland Altman analysis. Sensitivities of ABI, pPP, cPP, cfPWV, and aPWV for AOD were calculated. Results: Sixty-seven patients (35.8 % female, mean age 69, range 39–91 years) had a significantly higher aPWV than cfPWV (median 10.5 m/s (IQR: 8.8–12.65 m/s) vs. median 9.0 m/s (IQR: 7.57–10.55 m/s), p = 0.0013). There was no correlation between cfPWV and age (r = 0.311, p = 0.116). Bland Altman analysis revealed a mean difference of -1.04 (-2SD; -6.38 to + 2SD; 4.31). The sensitivities for AOD were 68.7 % for ABI, 61.2 % for aPWV, 40.3 % for cfPWV, 31.3 % for peripheral PP, and 10.4 % for central aortic PP (p < 0.001). Conclusions: Brachial-derived aPWV differs from the gold standard assessment (cfPWV), which may be underestimated in PAD due to atherosclerotic obstructions along the aorto-iliac segment. The sensitivities of noninvasive in vivo markers of AOD vary widely and tend to underestimate the actual presence of AOD.
Introduction
Arterial stiffness is an important and independent risk factor for cardiovascular events (CV) [1–3]. The assessment of arterial stiffness has gained substantial importance mostly in primary but also secondary prevention as it may guide therapy and predict the outcome [4–6]. A current large meta-analysis has reported that carotid-femoral pulse wave velocity (cfPWV) predicts future cardiovascular risk and improves risk classification in addition to established risk factors in low and intermediated risk patients in a primary prevention setting [7]. Aortic stiffness can be assessed in a number of ways. Carotid-femoral pulse wave travelling time currently is regarded as the gold standard and has been related to increased cardiovascular risks. However, aortic pulse wave velocity (aPWV), derived from [8, 9] carotid-femoral pulse wave travelling time, has not been studied extensively. PWV can be assessed in a routine clinical setting using a number of commercially available devices, making it an attractive in vivo marker of vascular disease. In addition, assessment of arterial organ damage (AOD), for example through ABI and PWV, has been proposed in asymptomatic individuals by the latest guidelines of the European Societies of Hypertension and Cardiology (ESH/ESC) [9]. A large body of evidence indicates that AOD plays a crucial role in determining the CV risk of asymptomatic individuals [8–10]. Vascular in vivo markers such as ABI index (ABI < 0.9), peripheral/central pulse pressure (pPP/cPP > 60 mmHg) and cfPWV (> 10m/s) are used to assess AOD according to guidelines [9, 10]. Atherosclerosis is irreversible. Early atherosclerosis is asymptomatic and associated with a markedly increased risk for coronary and cerebrovascular events [11]. Hence, peripheral arterial disease (PAD) is an overt AOD, which should be detectable by the assessment of the abovementioned markers of vascular disease. We hypothesized that cfPWV may not be appropriate to detect AOD in PAD due to atherosclerotic obstruction along the aorto-iliac segments. We therefore assessed PWV with two different commercially available devices and tested and compared sensitivities of PWV, cPP, pPP, and ABI to detect AOD in selected, well-defined patients with PAD.
Patients and methods
The study was conducted at the University Hospital of Zurich, which serves as a tertiary referral centre. ABI, PP (peripheral and central), and PWV (aortic and carotid-femoral) were assessed in consecutive patients with PAD who were either referred for clinical evaluation or follow-up. Only patients with chronic and stable PAD were eligible for the study. Inclusion criteria were colour-coded duplex sonography with plaques > 15 mm or a history of lower limb revascularization, which defined presence of PAD. Exclusion criteria were critical limb ischaemia (Fontaine IV), cardiac arrhythmia, and chronic inflammatory vascular disorders. Patients continued to take their regular medications. The presence of coronary and cerebrovascular diseases was defined by clinical history of either events or interventions. The study was approved by the local ethics committee and was conducted according to clinical practice standards [12]. Measurements were part of the standard care, including pulse volume recordings for oscillometric assessment of PWV and brachial blood pressure assessment for brachial derived PWV. The following data were collected: anthropometric data (height, weight), comorbidities and medications, major vascular risk factors (arterial hypertension, diabetes mellitus, dyslipidaemia, and smoking), peripheral systolic and diastolic blood pressures, heart rate, central blood pressures, ABI, cfPWV, and aPWV.
Pulse wave velocity
Pulse wave velocity was measured in all patients using a brachial cuff-based method (Mobil-O-Graph; I. E.M., Stolberg, Germany) and an oscillometric device (Vicorder; Skidmore Medical, Bristol, UK). Patients rested in a supine position for 10 Minutes in a quiet room. AOD was defined according to the ESH/ESC guidelines for the management of arterial hypertension as a PWV > 10 m/s [9]. All measurements were carried out in triplicate by the same vascular technicians and mean values of the triplicate measurements were calculated and used for analysis.
Mobil-O-Graph
The ARCSolver method, on which the Mobil-O-Graph measurement is based, performs pulse wave analysis based on oscillometric blood pressure measurements with a common blood pressure cuff. The method was developed by the Austrian Institute of Technology in Vienna and uses the pulse waves assessed at the brachial artery level [13]. Simplification of the signal processing is performed using a three level algorithm. In a first step, the single brachial pressure waves are verified for their plausibility, using pulse wave analysis and impedance wave separation. Thereafter, an aortic pulse wave is generated by means of a generalized transfer function [13–18]. The required flow is approximated by a model-based approach using methods described by Wassertheurer and Parragh [13, 19]. This is an operator-independent method for 24-hour ambulatory peripheral and central blood pressure and aortic PWV monitoring, invasively validated by Hametner et al. [15].
Vicorder
The Vicorder device allows measurement of carotid to femoral PWV. A collar is placed on the patient’s neck, equipped with a photoplethysmographic sensor, which is able to record carotid pressure waves. A second cuff is placed around the right upper thigh. The carotid and femoral waveforms are recorded simultaneously and estimate the transit time by means of the foot-to-foot method [16]. The foot point in the pressure wave was defined as the beginning of systole that was identified by an inbuilt algorithm that was centred on the peak of the second derivative of pressure [17, 18]. Path length was defined as the distance from the suprasternal notch to the top of the thigh cuff [17]. The cfPWV was calculated as path length divided by the transit time: cfPWV (m/s) = path length (m)/transit time (s).
Ankle-brachial index, peripheral and central pulse pressure
Standard brachial systolic and diastolic blood pressures on both arms with traditional cuff manometer were measured in triplicate according to the Riva Rocci method. Systolic ankle blood pressures of the anterior and posterior tibial artery on both legs were obtained using a hand-held 6-MHz Doppler probe. For each leg, ABI was calculated as the ratio of the highest ankle systolic blood pressure divided by the highest brachial systolic blood pressure, the lower ABI was taken as the study reference [20]. The AOD was defined as an ABI of < 0.9 or > 1.3 (incompressible tibial and peroneal arteries due to mediacalcinosis) according to the ESH/ESC guidelines for the management of arterial hypertension [9]. Pulse pressure represents the blood pressure amplitude, i. e. the difference of systolic blood pressure and diastolic blood pressure. We applied the peripheral pulse pressure and the noninvasive measurements of the central pulse pressure derived from the Mobil-O-Graph [15, 21]. The blood pressure (peripheral and central) was carried out in triplicate and mean values of the triplicate measurements were calculated and used for analysis. The AOD was defined according to the ESH/ESC guidelines for the management of arterial hypertension as a peripheral or central PP > 60 mmHg [9, 15].
Statistical analysis
Descriptive statistics for continuous variables are given as median and interquartile range (IQR). For categorical variables, results are presented as number and percentages. Age, ABI, and pulse pressure were considered as independent variables and PWV as a dependent variable. Univariate linear regression by Spearman Rank correlation was used to determine the association between cfPWV and age and pulse pressure. The two methods for PWV were analysed by univariate correlation and by Bland-Altman plot. Values of two-sided tests with p < 0.05 were considered statistically significant. The McNemar’s test was applied to test for sensitivity [22].
Difference of sensitivity between in vivo markers of vascular organ damage (ABI, cfPWV, aPWV, pPP, cPP) was assessed by Cochran’s Q test [23]. All analyses were performed using StatView software (Abacus, Berkeley, CA, U. S.A.) and R: a language and environment for statistical computing (the R Foundation for Statistical Computing, Vienna, Austria).
Results
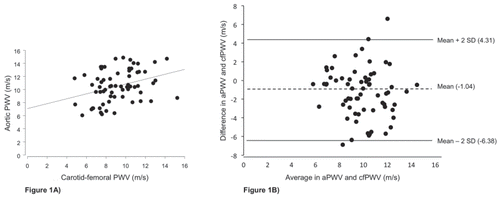
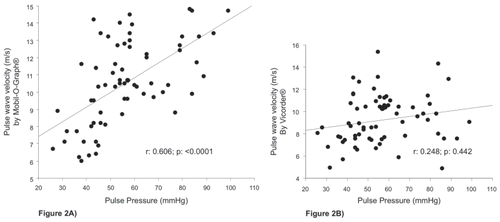
Sixty-seven patients (24 women (35.8 %)) with PAD and a mean age of 69 years (range 39–91 years) were studied. The characteristics of the study population are summarized in Table 1. The PWV from the Mobil-O-Graph (median 10.5 m/s; IQR 8.8–12.7 m/s) were significantly different (p = 0.0013) from the Vicorder (median 9 m/s; IQR 7.6–10.6 m/s). Figure 1 shows the univariate correlation and the Bland-Altman plot for the two devices. The mean difference was -1.04 (-2SD, -6.38; + 2SD, 4.31). Correlation with age was found for aPWV (Mobil-O-graph) with r = 0.935 (p = < 0.0001) but not for the cfPWV (Vicorder) (r = 0.311 (p = 0.116)). Figure 2A/2B shows the correlation between PWV and peripheral pulse pressures. The correlation coefficients were r = 0.606 (p = < 0.0001) for pulse pressure and aPWV (by Mobil-O-Graph) and r = 0.248 (p = 0.442) for pulse pressure and cfPWV (by Vicorder).

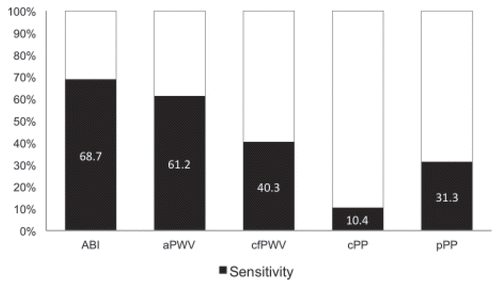
Twenty-one (31 %) of a total of 67 patients had a normal ABI ranging from 0.9 to 1.3 with a median of 0.98 (IQR 0.93–1). Forty-one (62 %) patients had an ABI lower than 0.9 (median 0.6 (IQR: 0.46–0.74), and five (7 %) patients had mediacalcinosis with a median ABI of 1.4 (IQR: 1.38–1.4). Sensitivities for the presence of AOD in relation to each of the in vivo markers of arterial organ injury are shown in Figure 3. Sensitivities to detect AOD in PAD patients ranged from 10.4 % (central pulse pressure) to 68.7 % (ABI). The carotid-femoral PWV, the current gold standard, had a sensitivity of 40.3 % for AOD. The sensitivities for AOD between aPWV and cfPWV differed significantly (p = 0.043) with better sensitivity of aPWV for AOD. The difference in sensitivities between aPWV and ABI were not significant (p = 0.3173). After correction for multiple testing, the differences between the sensitivities of aPWV and cfPWV remained significant (p = 0.008). Cochran’s Q test confirms that there is strong evidence that the five diagnostic tests do not have the same sensitivity (p < 0.001) [23].
Discussion
In this study we determined the sensitivities of five relevant arterial disease indices or in vivo markers of AOD that are all easily applied, noninvasive, and in part automated in their use. We found that currently used in vivo markers of AOD have a much lower sensitivity to detect vascular damage in patients with PAD than expected. This may result in insufficient detection or underdiagnosed vascular damage in PAD using each measurement modality. These in vivo markers have gained great attention as screening tools for AOD mostly in asymptomatic patients with cardiovascular risk factors in a primary care setting. All parameters are based on blood pressure relations (ABI, central and peripheral PP) or pulse wave characteristics (aortic and carotid-femoral PWV). As expected, ABI revealed the highest sensitivity for AOD. Accordingly, the difference between upper to lower extremity blood pressure per se is the noninvasive diagnostic tool of choice for PAD. We also included PAD patients with an ABI within the normal range following lower limb angioplasty and found that ABI was not pathologic and hence not sensitive to AOD. Unexpectedly, cfPWV had a low sensitivity for AOD in PAD and even lower than aPWV. As a consequence, aPWV and cfPWV measurements did not correlate, revealing higher values for PWV derived from the brachial artery (aPWV). This might be explained by the fact that the brachial artery is mostly free of atherosclerotic plaque and thus pulse wave analysis may be more accurate. Unexpectedly, cfPWV was not associated with age. This may be explained by the fact that atherosclerosis and obstructive lesions along the aortic and iliac arterial segments cause delay in systolic upstroke of the wave at the femoral level resulting in a false low cfPWV [24]. Therefore, cfPWV can only be reliably assessed if relevant atherosclerotic disease is absent between the carotid and femoral artery. Thus, a comprehensive vascular assessment including imaging prior to a simple cfPWV assessment, especially in elderly patients and those with cardiovascular risk factors, is necessary due to the high prevalence of PAD. Alternatively, measurements could be performed with the Mobil-O-Graph for the brachial measurement using the ARCSolver, a novel method to noninvasively estimate aortic PWV from a single brachial cuff waveform [13, 25, 26]. Hametner and Wassertheurer showed a significant linear correlation between the noninvasive assessment and the intraaortic pressure measurement [15]. This suggests that the combination provides an easily gained approximation for aortic PWV with promising results compared to other methods and that it might be of use in PAD [23]. Other studies, which investigated PWV in PAD using the SphygmoCor device, support our findings. Brand et al. investigated 136 patients with critical limb ischaemia and 194 controls. They demonstrated that PWV is markedly lower in patients with critical limb ischaemia (PWV = 5.72 m/s) when compared to healthy controls (PWV = 8.62m/s). Similarly, there was no correlation between PWV and age [24]. In contrast, Catalano et al. reported higher PWV assessed by SphygmoCor in PAD patients than in the control group (11 ± 3 m/s vs. 9.8 ± 1.8 m/s), which negatively correlated with ABI in the PAD group and had no correlation with age (r = 0.13, p = 0.06) [27]. Although these findings can be considered conflicting, they indicate that cfPWV should be assessed with caution in presence of PAD.
Other parameters that are derived from blood pressure measurements are the central aortic and peripheral pressure amplitudes. Arterial stiffening resulting from aortic atherosclerosis or loss of Windkessel function increases systolic arterial blood pressure and decreases diastolic blood pressures, resulting in an increase in blood pressure amplitude, which is a recognized pressure pattern in PAD [28, 29]. Despite the fact that the pathophysiology of these changes is well understood and documented, the suggested cut-off at > 60 mmHg in amplitudes was not sufficient to identify AOD in the majority of our population, irrespectively of whether values were calculated as peripheral or central blood pressure amplitudes. In our population, the sensitivities of cPP or pPP higher than 60 mmHg were as low as 10 % and 30 %, respectively.
The findings of the present study have a number of relevant implications for both clinical and scientific evaluation of in vivo arterial disease markers. First, the results show that a marker currently considered as gold standard (cfPWV) for disease detection has some drawbacks in the presence of atherosclerotic arterial diseases. Atherosclerotic vascular changes along the aortic and iliac arterial conduit may result in false low velocities, suggesting less or even absence of AOD. Even ABI, the gold standard assessment for PAD, may be not sensitive for atherosclerotic damage in case of non-significant arterial stenosis. In this case, AOD can only be detected by noninvasive or invasive imaging, such as duplexsonography, intraarterial angiography or computed angiograms. In our patient selection, we diagnosed PAD prior to inclusion in the study. Second, our findings indicate that cut-off values presented in current guidelines for the management of arterial hypertension, that recommend assessment of cfPWV in hypertensive patients [9], can only be advocated after exclusion of PAD and suggest that single assessment such as cfPWV should not be performed in settings without proper medical knowledge or training. Third, devices for measurement of PWV differ in their sensitivity to detect AOD and some methods might have advantages over the current gold standard.
Limitations
The sample size of our study was limited. More work is needed to evaluate the differences of cfPWV dependent on the stage and localization of the stenosis/obstruction.
Conclusions
In conclusion, in vivo markers of AOD do not offer satisfying sensitivities in presence of peripheral arterial disease and even the current gold standard to assess PWV has to be used with caution, specifically in PAD patients. Given that advanced age is an independent determinant of generalized atherosclerosis, markers of AOD in elderly patients have to be evaluated in relation to potential presence of an asymptomatic peripheral arterial disease due to its high prevalence in the aged population.
Acknowledgments
This study has been supported by the Swiss Heart Foundation.
There are no conflicts of interest existing.
Literature
Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121(4):505–11.
. Arterial Stiffness and Wave Reflection: Biomarkers of Cardiovascular Risk. Art Res. 2009;3(2):56–64.
Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43(6):1239–45.
Regression of Left Ventricular Mass in Hypertensive Patients Treated With Perindopril/ Indapamide as a First-Line Combination. Am J Hypert. 2004;17(8):660–7.
. Arterial stiffness, hypertension, and rational use of nebivolol. Vasc Health Risk Managem. 2009;5:353–60.
. Reduced arterial stiffness may contribute to angiotensin- converting enzyme inhibitor induced improvements in walking time in peripheral arterial disease patients. J Hypertens. 2008;26(5):1038–42.
Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. JACC. 2014;63(7):636–46.
Expert consensus document on arterial stiffness: methodological issues and clinical applications. Europ Heart J. 2006;27(21):2588–605.
2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Europ Heart J. 2013;34(28):2159–219.
Pulse Pressure : A Predictor of Long-term Cardiovascular Mortality in a French Male Population. Hypertension. 1997;30(6):1410–5.
Severity of peripheral arterial disease is associated with aortic pressure augmentation and subendocardial viability ratio. J Clin Hypertens. 2012;14(12):855–60.
International Conference on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human use. Guideline for Good Clinical Practice. J Postgrad Med.1996.
. Modeling arterial and left ventricular coupling for non-invasive measurements. Simul Model Pract Theory. 2008;16(8):988–97.
. Effects of Different Blood Flow Models on the Determination of Arterial Characteristic Impedance. Math Comp Model Dyn. 2013;19(4):319–30.
. Oscillometric estimation of aortic pulse wave velocity: comparison with intra-aortic catheter measurements. Blood Press Monit. 2013;18(3):173–6.
. Mathematical methods for determining the foot point of the arterial pulse wave and evaluation of proposed methods. Inform Tech Control. 2005;34(1):29–36.
. Validity and repeatability of the Vicorder apparatus: a comparison with the SphygmoCor device. Hypertens Res. 2009;32(12):1079–85.
. Oscillometric carotid to femoral pulse wave velocity estimated with the Vicorder device. J Clin Hypertens. 2013;15(3):176–9.
. Influence of an Asymptotic Pressure Level on the Windkessel Models of the Arterial System. IFAC-PapersOnLine. 2015;48(1):17–22.
. Ankle–brachial index for assessment of peripheral arterial disease. New Engl J Med. 2009;361(e40).
24-h ambulatory recording of aortic pulse wave velocity and central systolic augmentation: a feasibility study. Hypertens Res. 2012;35(10):980–7.
. Does McNemar’s test compare the sensitivities and specificities of two diagnostic tests? Stat Meth Med Res. 2017;26(1):142–54.
. The Comparison of Percentages in Matched Samples. Oxford Journal Oxford University Press. 1950;Vol. 37, No. 3/4: 256–66.
. A mismatch between aortic pulse pressure and pulse wave velocity predicts advanced peripheral arterial disease. Eur J Vasc Endovasc Surg. 2013;46(3):338–46.
A new oscillometric method for pulse wave analysis: comparison with a common tonometric method. J Hum Hypertens. 2010;24(8):498–504.
. Effect of verapamil on home self-measurement of blood pressure and heart rate by hypertensive patients. Verapamil-Frequency Research Group. Blood Pres Monit. 2000;5(1):23–30.
Increased aortic stiffness and related factors in patients with peripheral arterial disease. J Clin Hypertens. 2013;15(10):712–6.
. Contribution of the Arterial System and the Heart to Blood Pressure during Normal Aging – A Simulation Study. PloS one. 2016;11(6):e0157493.
Aortic root dimension and arterial stiffness in arterial hypertension: the Campania Salute Network. J Hypertens (LWW Journals). 2016;34(6):1109–14.



