ESVM Guideline on peripheral arterial disease
ESVM Board Authors
Vinko Boc* (Slovenia), Marianne Brodmann (Austria), Patrick H Carpentier (France), Ali Chraim* (Ukraine), Caitriona Canning (Ireland); Evangelos Dimakakos* (Greece), Anders Gottsäter (Sweden), Christian Heiss (Germany), Lucia Mazzolai (Switzerland), Juraj Madaric* (Slovakia), Dan Mircea Olinic* (Romania), Zsolt Pécsvárady* (Hungary), Pavel Poredoš* (Slovenia), Isabelle Quéré (France), Karel Roztocil (ESVM President, Czech Republic), Agata Stanek (Poland), Dragan Vasic* (Serbia), Adriana Visonà (Italy), and Jean-Claude Wautrecht* (Belgium)
ESVM Country Society Authors
Miroslav Bulvas (Czech Republic), Mary-Paula Colgan (Ireland), Walter Dorigo (Italy), Graeme Houston (UK), Thomas Kahan (Sweden), Holger Lawall (Germany), Isak Lindstedt (Sweden), Guillaume Mahe (France), Romeo Martini (Italy), Giles Pernod (France), Stanislaw Przywara (Poland). Marc Righini (Switzerland), Oliver Schlager (Austria), and Piotr Terlecki (Poland)
*Indicates ESVM Board members who were also nominated by their Country Society as reviewers.
The European Society of Vascular Medicine (ESVM) was founded in Paris in 2012 as a confederation of already existing and emerging European societies of vascular medicine. At the founding congress of the new society in Potsdam, Germany in 2015 the different national societies sent experts to a panel to draft an ESVM Guideline on Peripheral arterial disease (PAD). After reviewing and discussing the already existing national guideline of the different countries, the panel decided to consider the latest German S3 Guideline on PAD from November 2015 [1] as the backbone of the new ESVM PAD Guideline, update where appropriate and supplement with data from rigorous literature searches. Permission from the German Society was given.
The ESVM acknowledges the significant and valuable aid from the German Angiology Society and their generosity in allowing their own Guideline to form the basis of this Guideline.
Thus, the German S3 guideline [1], which is a national intersociety consensus guideline – with multiple different participating specialties – was translated into English, reviewed and shortened or adapted according to consensus achieved among ESVM experts and some new chapters and new recommendations/statements added.
The clear focus of the new ESVM Guideline is – in the spirit of the Society – the medical and endovascular aspects of the diagnosis and therapy of PAD. The aim of the ESVM guideline process was to harmonize and optimise the content and the principal statements between the different national member societies of the ESVM. Thus, a draft of the new guideline was presented to all national European societies for revision and comment prior to finalization. The literature has been reviewed to January 2019.
Summary of consensus process
For the German guidelines, a total of 27 medical societies/organisations, two support groups and the German Pension Fund (Deutsche Rentenversicherung Bund) received written invitations to participate in the original guideline. A total of 24 societies/organizations had responded by the end of 2012, altogether signaling their interest in collaboration.
For this current ESVM guideline, the 19-member states of the ESVM contributed, with a small writing group taking the lead. All 19 angiology societies were given time to review the new common guideline and formally endorsed its content. The key issues were formulated and agreed upon at consensus meetings and were gradually elaborated by the ESVM PAD guideline working group to harmonize standards of care within the ESVM member countries. Then, the content was further discussed and accredited in final format by the national angiology/vascular medicine societies.
Timeline of work
- •Guideline Committee approval of subject matter, permission from German Society of Angiology to use German Guideline as basis for ESVM Guideline (Rome May 2016)
- •Allocation of writing group (Rome 2016), translation from German, literature search (May to Nov 2016)
- •Draft 1: Presentation of draft 1 to Guideline Committee and ESVM Board by nominal group process, (Hamburg November 2nd 2016)
- •Draft 2: Full day Consensus Meeting (Hamburg November 3rd 2016)
- •Draft 3: Circulation of draft 3 to ESVM Board taking into account Consensus Meeting comments,
- •Draft 4: Presentation of draft 4 to ESVM Congress (Prague, March 2018) at a Consensus Meeting,
- •Draft 5: Circulated to the ESVM Board
- •Draft 6: Circulated and approved by the ESVM Board, after taking into account further ESVM Board comments, as ready to be shared with Country Societies, by nominal group process (May 2018)
- •Draft 7: circulated to all Vascular Medicine/Angiology Society representatives/authors for comments (May to Oct 2018).
- •Draft 8: Circulated to ESVM Board and Society Representatives, considering Society Representatives comments (Oct 2018)
- •Draft 9: ESVM Board meeting (Frankfurt Oct 2018) by nominal group process
- •Draft 10: Literature update via ESVM Guideline Committee (Jan 2019)
- •Draft 11: Incorporation of final comments from all, penultimate draft, circulated to ESVM Board and Country Society Representatives for final approval and sign off (May to June 2019)
- •Draft 12: Penultimate version circulated to ESVM Board, Guideline Committee and Country Reviewers
- •Draft 13: Final version circulated as per version 12 and approved
1.1 Objective
The objective of the present guideline is to provide evidence-based, comprehensive and optimal care recommendations for patients with atherosclerotic peripheral artery disease (PAD) of the lower limbs. The guideline is meant to aid medical personnel and patients in making decisions regarding the optimal diagnostic and therapeutic measures for patients with PAD, and to aid with action and decision routes, which may be modified in justified cases. Guidelines established by scientific medical societies are not legally binding for physicians and may thus neither cause liability nor release physicians from liability. What legally constitutes a medical standard in the treatment of a given individual can only be determined individually. The present guideline thus does not relieve physicians from their obligation to individually manage their patients by appraising their patients’ overall situation.
The present guideline aims to compile the most significant evidence and information on the treatment of peripheral arterial disorders from various specialties to offer the reader reliable assistance in everyday practical clinical life.
1.2 Which patients does this guideline refer to?
The guideline refers to adults of any age with asymptomatic or symptomatic peripheral arterial circulation disorders due to atherosclerosis. It is also valid for patients at a markedly increased PAD risk, e.g. atherosclerosis patients with coronary artery disease, carotid stenosis, renal impairment, diabetes mellitus or cerebrovascular disease. The guideline covers all areas of epidemiology, diagnostics, treatment and follow-up care for patients with PAD bar the surgical options.
The guideline does not apply to children. Treatment strategies for non-atheromatous causes of peripheral artery occlusion processes (vasculitis, dissection, giant-cell arteritis, fibromuscular dysplasia, radiogenic stenoses, and entrapment syndromes) are to be distinguished from atherosclerotic stenoses/occlusions and they are not the focus of the present guideline.
1.3 Guideline users
The guideline is addressed to all concerned with the care and treatment of patients with PAD. The addressees include physicians, and allied health professionals in outpatient/in-patient care and rehabilitation medicine who care or treat patients with PAD. The guideline is also intended to serve as a source of up-to-date information for public-health institutions, and government policy.
1.4 Participation of interest groups
1.4.1 Organization, financing, and editorial freedom
This Guideline was financed by the ESVM. No monies from Industry were received for the preparation of this guideline. All the current guideline group members disclosed potential conflicts of interest in writing and these are present online in Appendix 1.
1.5 Review and selection of scientific documents (evidence-based)
National and international guidelines were systematically reviewed in the guideline International Network (http://www.g-i-n.net/) database for national and international guidelines that had been published under the search terms “PAD”, “peripheral arterial disease”, “peripheral artery disease” and “claudication “and “critical limb ischemia”. Importance was attached to the systematic development of, and comprehensible evidence base for, the given recommendations. Secondly, the literature was scanned for further new evidence-based findings that would modify the German guideline including more recent publications to January 2019.
1.6 Recommendations and levels of evidence
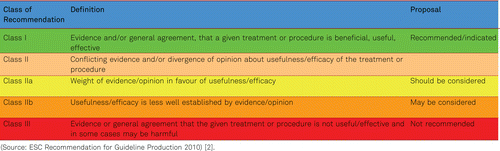
For the description of the scientific evidence and the degree of recommendation we adopted our systematology on the recommendations of the European Society of Cardiology and the European Society for Vascular Surgery [2]. Thus, we describe the level of evidence in three categories, from A (best level of evidence based on multiple randomized clinical trials) to C (consensus of opinion of the experts or small studies or registries. Based on these different levels of evidence we formulated our recommendations in the generally accepted way of type I (recommended) to III (not recommended) statements (Table 1.6).
2 Definition and epidemiology
2.1 Definition
Peripheral arterial disease (PAD) affecting the lower limb occurs where there is a blood circulation disorder of the arteries that supply the limbs, which may be partial (due to a stenosis) or complete (due to an occlusion). In approx. 95 % of cases, chronic PAD is caused by atherosclerosis. It is a complex medical condition, which may be asymptomatic in its early stages, although that may affect all arterial vascular regions of the body. In addition to the large peripheral vessels, smaller vessels supplying the skin and muscles are often affected. Acute severe peripheral circulatory disorders are rarer than the chronic form, developing from acute embolic or atherothrombotic vascular obstructions such as plaque rupture. The following guideline recommendations address acute and chronic arterial circulatory disorders in the lower limbs distal to the abdominal aorta.
Inflammatory, genetic, and traumatic causes (approx. 5 % of PAD cases) become less frequent with increasing age, while embolic events (cardiac or arterio-arterial) become more frequent [3].
Myocardial infarction, stroke, and PAD are different manifestations of atherosclerosis [4]. The most severe form of PAD is tissue death / necrosis with the threat of amputation of the affected limb. This Guideline deals with peripheral arterial disease affecting the lower limb only.
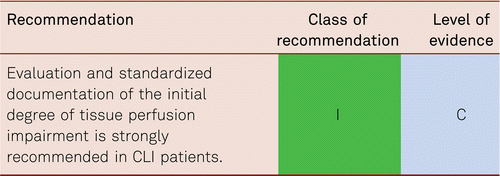
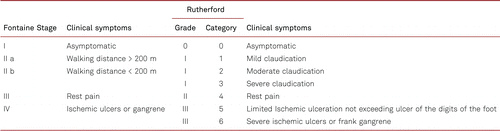
In Europe, the Fontaine staging system is applied to clinically classify PAD in terms of symptoms. The Rutherford classification is more conventional in the USA and international science, and distinguishes between 3 grades and 6 categories, which makes the categorization of the severity of the disease more precise. Table 2.1 shows the classification of PAD according to Fontaine stages and Rutherford grades and categories. The clinical stages are indicated with the terms “intermittent claudication” (Fontaine stage II) and/or “critical limb ischemia” (CLI) in later Fontaine stages III and IV.
2.2 Ankle-brachial pressure index
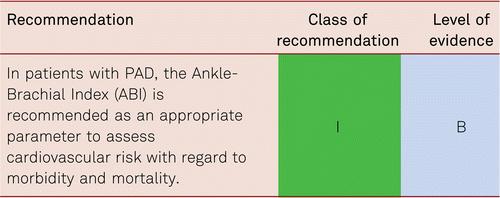
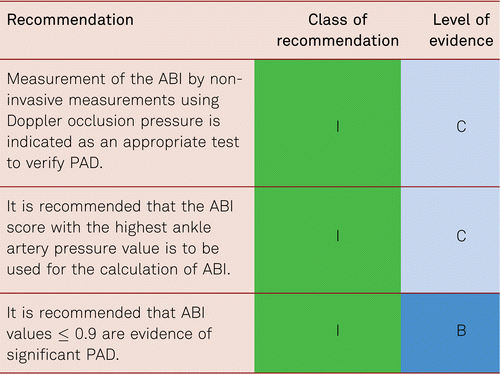
The prevalence of asymptomatic PAD among the general population can only be estimated by non-invasive methods of investigation. Most frequently this is detected by the ankle-brachial pressure index (ABI) measured by non-invasive Doppler pressure measurement (also see Chapter 3.2, Diagnostics). The cut-off value for the diagnosis of PAD is an ABI value ≤ 0.9. The sensitivity of an ABI value of ≤ 0.9 for the presence of at least a 50 % vascular stenosis (verified by the gold standard angiography) is almost 95 % at rest, with a specificity of nearly 100 % [5].
Systematic ABI measurements following stress tests increase the detection and thus the prevalence of PAD by approx. 30 %. An ABI decrease by 15 to 20 % following walking stress – compared with baseline values at rest – is indicative of PAD [6, 7].
2.3 Epidemiology
2.3.2 Prevalence and incidence
Numerous epidemiological studies based on objective research techniques (usually ABI measurements) have shown an overall 3 to 10 % prevalence of PAD within the population. The prevalence of symptomatic intermittent claudication increases from 3 % among 40-year-old patients to 6 % among those aged 60-65. From age 70 the prevalence is seen to increase to 15 to 20 % [8, 9]. In 2010, the global prevalence of PAD (ABI ≤ 0.9) was mathematically estimated from the data of a systematic review to be worldwide 202 million. Within the EU between 2000 and 2010, the incidence increased by 28.7 % in low- and medium-income countries and by 13.1 % in high-income countries [10].
The ratio between patients diagnosed as asymptomatic by ABI and those presenting with symptomatic claudication (who mostly have decreased ABI values) is approx. 4:1 irrespective of age [11].
Multisite atherosclerotic artery disease is common and associated with a worse outcome, as reviewed elsewhere [12]. Thus, in people with PAD (ABI < 0.90), CAD was present in 25–70 %, carotid artery stenosis (> 70 %) in 14–19 %, and renal artery stenosis (> 75 %) in 10–23 %. In patients with carotid artery stenosis, PAD was present in 18–22 %, whilst patients with CAD were reported to have PAD in 7–16 % [12]. Compared to people with no PAD, the prevalence of CAD is two- to four-fold higher in PAD patients, and the severity of PAD is associated to the prevalence of CAD. Conversely, left main coronary artery stenosis and multivessel CAD are independent predictors of PAD, and patients with PAD exhibit more advanced coronary atherosclerosis. Accordingly, coexisting PAD in patients with CAD is associated with a worse outcome.
Also, cardiac conditions other than CAD are common in PAD patients [12]. Compared to a general population, left ventricular dysfunction is at least two-fold more prevalent in patients with PAD, matched for age and sex. PAD increases the risk for incident heart failure, at least in people with no prevalent CAD. A multivariable-adjusted population-attributable risk for incident heart failure with an ABI < 1.00 was 6 %, compared with 8 % for CAD, 15 % for hypertension, and 14 % for diabetes [13]. Furthermore, the presence of PAD in patients with heart failure independently predicts hospitalizations and death. Patients with PAD have an increased risk for incident atrial fibrillation. Also, the presence of atrial fibrillation in patients with PAD is associated with more severe forms (assessed by the Rutherford classification) and with an increased cardiovascular morbidity and mortality.
In the prospective, non-interventional nationwide epidemiological trial on Ankle-Brachial Index (Get-ABI Study), every fifth (21 %) of 6,880 patients aged 65 or older had an ABI of < 0.9 or clinical manifestation of PAD [14]. Investigating 4,814 subjects aged 45 to 75 in the general population, the Heinz Nixdorf Recall Study identified an ABI of < 0.9 in 6.4 % (men) and 5.1 % (women), increasing to 8.2 % and 5.5 % when asymptomatic forms of PAD were additionally taken into consideration [15].
The PAD Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program produced prevalence data of patients at risk (aged ≥ 70 years or 50- to 69- year-old smokers or patients with diabetes) in primary care. In this study, 29 % of the overall population showed a decreased ABI or manifest PAD [16]. In diabetes patients in the POPADAD study over the age of 40 years this was 20 % [17].
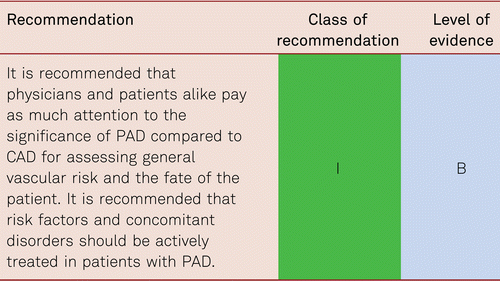
According to the current literature, however, general screening of asymptomatic patients is not recommended at present, the prevalence among low-risk groups being 1 to 4 % and approximately 17–20 % in mixed-risk populations [17, 18]. Although, asymptomatic patients have a higher than normal risk of cardiovascular events, this guideline does not deal with recommendations for the management of these subjects but focusses on the key area of risk reduction in symptomatic patients.
2.3.2.1 Gender prevalence
Claudication is more frequent among men in younger age groups, but there is practically no gender-specific differences in older age groups. Indeed, in the GetABI Study, PAD prevalence was seen to be higher in women than in men after the age of 75 [9]. At the time of diagnosis of PAD, women are older, more frequently obese, have diabetes, and more frequently present with CLI and vascular obstruction; men are more frequently smokers [19, 20].
However, preliminary indications suggest a gender-dependent distribution pattern of PAD – with cumulative femoropopliteal and multilevel disease in women and a more frequent infrapopliteal distribution pattern among men – this requires further confirmation in larger-scale study populations [21].
2.3.2.2 Prevalence in different ethnic groups
Links with different ethnic populations such as those of Afro-Caribbean decent (non-Hispanic origins) have been seen to increase the risk of PAD by more than twofold, which cannot be exclusively explained on the basis of an increased presence of other risk factors, such as hypertension and diabetes [22]. These differences in PAD prevalence have recently been corroborated by the Genetic Epidemiology Network of Arteriopathy (GENOA) study [23].
2.4 Cross risk of atherothrombotic events
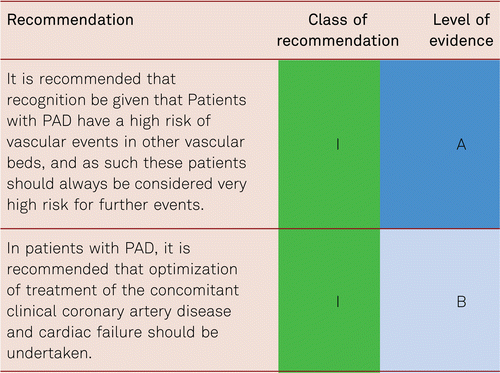
In the presence of an atherothrombotic disease in one vascular bed (e.g. PAD), patients are at high risk of cardiovascular morbidity and mortality in other vascular beds. Table 2.2 summarizes the “cross risk” between different atherothrombotic manifestations. PAD patients, having experienced one atheromatous disease, are thus at a clearly increased risk for further cardiovascular events, including myocardial infarction and ischemic stroke.

2.4.1 Concomitant PAD and coronary artery disease and/or cardiac failure
2.4.1.1 Coronary artery disease (CAD)
The occurrence of both PAD and CAD together is frequent and easily overlooked due to a limitation of walking distances by angina pectoris or dyspnea, or vice versa. Such a combination severely worsens prognosis of the patients compared with those with a single manifestation [28].
The IPSILON study, a French cross-sectional study in primary care patients, detected PAD in 26.6 % of 1,340 patients with CAD without known manifestation of PAD by means of the ABI [29]. An investigation with simultaneous peripheral and coronary angiography in patients with claudication or CLI identified prevalent CAD (≥ 50 % coronary stenosis in coronary angiography) among 67 of 107 patients (62 %). The presence of diabetes mellitus further increased the likelihood of coincidental CAD and especially coronary multivessel disease in patients with PAD [16, 30]. In patients from the Reduction of Atherothrombosis for Continued Health (REACH) registry, fatal and nonfatal events increased from 13 % in patients with CAD alone to 23.1 % in those with coincidental CAD and PAD after one year of observation [31].
2.4.1.2 Heart failure
In a sub-study of the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) trial in patients with heart failure with reduced left ventricular systolic function (HFrEF), multivariate analysis showed patients with CLI (637 of 5,011 patients, 12.7 %) to have both an increased mortality risk (HR 1.36; 95 % CI: 1.19–1.56) and an increased risk for fatal and nonfatal MI (time to first event HR 1.67, 95 % CI: 1.24–2.27) compared to patients without concomitant PAD [32]. In addition, cardiac failure impairs peripheral blood circulation due to decreased cardiac output, as well as the patency rates after endovascular interventions.
Patients with both disorders are physically less resilient and show less improvement through physical training. This was shown by an investigation within the “Heart Failure: A Controlled Trial Investigating Outcomes of exercise traiNing” (HF-ACTION) in patients with ejection fraction ≤ 35 % and cardiac failure NYHA II to IV. Patients with concomitant PAD (157 of 2,331, 6.8 %) showed less improvement in a structured physical exercise program than patients without concomitant PAD. This disorder was shown to be an independent predictor of mortality or hospitalization (HR 1.31, 95 % CI: 1.06–1.62) [33].
Following endovascular intervention, participants with concomitant HFrEF (EF < 40 %) showed poorer primary 1-year patency rates (43.2 % vs. 56.6 %) compared with patients with an EF of > 40 %, and similar differences were seen in secondary patency rates. Finally, limb preservation was poorer in patients with coincidental cardiac failure [34].
2.4.2 PAD and diabetes mellitus
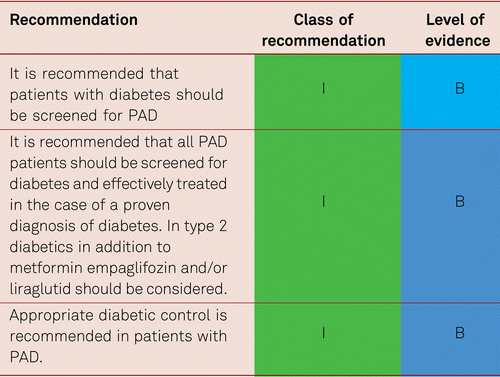
Regardless of type, diabetes mellitus is associated with an increased risk of peripheral atherosclerosis. A systematic review of risk factors for PAD from 34 trials conducted since 1997 estimated the pooled relative risk of PAD due to diabetes mellitus with an odds ratio of 1.88 (95 % CI: 1.66–2.14) [10]. In particular, diabetes increases the probability of distal disease in PAD and CAD multi-vessel disease, which more frequently results in the need for coronary and/or peripheral bypass surgery compared with patients without diabetes [35].
In patients with type 2 diabetes, the rate of cardiovascular events increases with elevated HbA1c levels. A systematic review estimated the pooled relative risk per 1 % increase in HbA1c for overall mortality to be 1.15 (95 % CI: 1.10–1.20), for cardiovascular disease to be 1.15 (95 %CI: 1.10–1.20), for the development of cardiac failure to be 1.11(95 % CI: 1.06–1.17), and for the emergence of PAD to be 1.29 (95 % CI: 1.18–1.40) [36]. In turn, a Danish case-control study failed to determine the benefit of intensified diabetes therapy compared to conventional treatment in terms of the de novo incidence of PAD after 6 years [37].
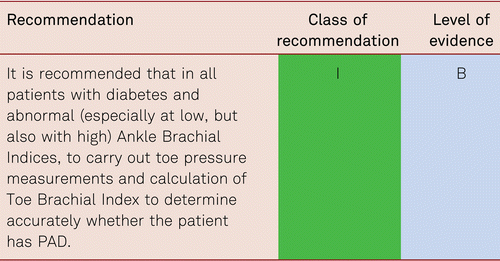
All patients with diabetes mellitus are at risk of developing diabetic foot syndrome should undergo an ABI and preferably toe pressure measurement alongside clinical foot examinations [38]. In patients with elevated ABI values, additional toe pressure measurement is mandatory, as false elevated ABI values occur in diabetes due to vessel stiffening/calcification [39].
2.4.3 PAD and concomitant renal failure
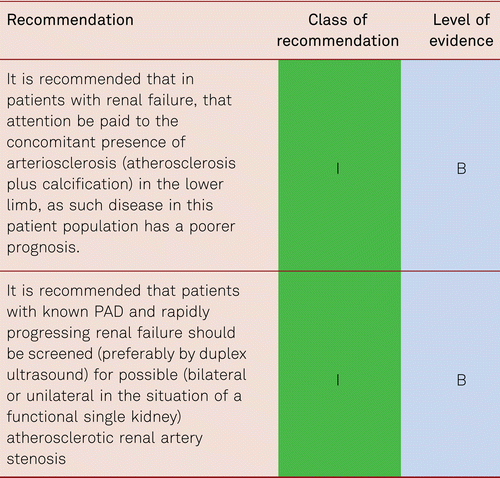
Concomitant renal failure (chronic renal failure (CKD)) impairs the prognosis of cardiovascular disorders overall and lowers the rate of amputation-free survival. In a prospective cohort study, 104 patients with CKD were followed up for 3 years following CLI therapy, survival and limb preservation were recorded. Treatment had consisted of bypass surgery (55 %), endovascular intervention (45 %), conservative treatment (22 %), or primary amputation (9 %). Subjects with cardiac comorbidity and renal failure with serum creatinine levels > 2 mg/dl showed a poorer outcome in terms of amputation-free survival (HR3.68, 95 % CI: 1.51–8.94) [40, 41]. Arteriosclerosis may complicate this, where calcium deposits appear in the media causing vessel stiffening. Carotid artery disease is very common in this group.
2.5 Progression of PAD and prognosis
2.5.1 Asymptomatic PAD
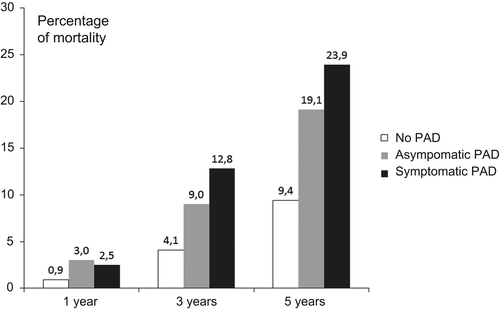
Both pathologically lowered, and pathologically increased ABI values are predictors of cardiovascular morbidity and mortality [42–45]. There is a direct correlation between ABI and CV events and death. The lower the ABI, the higher the cardiovascular morbidity and mortality. The exception to this is in diabetes where vessel stiffening may give a false high reading (Figure 2.1).
2.5.2 Symptomatic PAD
Symptoms improve spontaneously in approximately one quarter of patients with claudication. The disorder remains unchanged in approximately one third to one half of all subjects and deteriorates in about one quarter. The fate of patients with claudication is determined by cardiac and cerebral events. The risk for CLI is very low among patients with claudication and only 2 % experience an amputation within 10 years of PAD diagnosis [7].
Thirty years ago, Wolfe reported a 20 % 1-year mortality rate in CLI [46]. In the recent French “Cohorte des Patients Artériopathes” (COPART) registry with 940 patients, the 1-year mortality rate among patients with stable intermittent claudication (IC) was 5.7 % compared to 21.1 % in patients with CLI. The rate was 28.7 % among those presenting with ulceration [47]. In the “Bypass versus Angioplasty in Severe Ischemia of the Leg” (BASIL) study, 1- and 3-year amputation-free survival amounted to 70 % and 55 %, respectively, in the overall group of participants with CLI, with a 1-year mortality rate of approximately 20 % [48].
A recent meta-analysis by Sigvant et al, described that symptomatic PAD subjects continue to have higher 5 year cumulative CV mortality than the reference population, 13 % versus 5 %. During follow up 21 % of IC patients were diagnosed as having critical limb ischemia, with 4–27 % undergoing amputations [49].
Prognosis is even worse in patients with diabetes mellitus. An Italian study identified a 12-month mortality rate of 26 % in patients with diabetes and 12 % in patients without diabetes; the major amputation rate was 50 % after one year [50].
The “Reduction of Atherothrombosis for Continued Health” (REACH) registry [51] is a multinational database registering the frequency of atherothrombotic disorders and atherothrombosis-associated risk factors in clinical practice. With some 68,000 patients in 44 countries, it is the largest atherothrombosis registry in geographical terms and also by its patient sample size. After one year of follow-up, subjects with symptomatic PAD already showed a significantly higher mortality rate than those with coronary artery or cerebrovascular disease: 2.4 % in PAD patients vs. 1.8 % in CAD. The annual amputation rate was 1.3 % and the annual intervention rate for vascular interventions was 10 % among PAD patients [31].
The annual rates of major amputation in Europe have shown a decrease, reducing from 4.6 % in 2005 to 3.5 % in 2009. At the same time, the rate of minor amputations increased minimally from 5.0 to 5.1 %. Intra-hospital mortality among patients with claudication remained stable (2.2 %) and dropped from 9.8 % to 8.4 % among CLI patients in this period [52]. Similarly there is an improved use of preventative measures.
In terms of Rutherford categories, the 4-year mortality risk projected from the Kaplan-Meier model increases from 18.9 % (grade 1 to 3), to 37.7 % (grade 4) and 52.2 % (grade 5), and to 63.5 % (grade 6). The 4-year amputation risk estimated with the same model for grade 1 to 3, 4, 5, and 6 increases from to 12.1 % to 35.3 % and finally to 67.3 % [53].
2.6 Under-treatment of PAD patients
Many studies have reported an under-treatment of patients with PAD, especially in direct comparison with patients with CAD [54]. The US PARTNERS program demonstrated medical under-treatment in this patient population [16]. The GetABI study also showed PAD patients to be under-treatment in comparison with other atherothrombosis patients (CAD; stroke). Two out of three patients with CAD were given antiplatelet drugs, but only approximately half of the patients with PAD. The situation was similar with regard to lipid lowering with statins: 46 % of CAD patients, yet merely 23 % of patients with symptomatic PAD, were treated with statins. The difference between CAD and PAD was even more pronounced in the prescriptionof beta blockers [55, 56] (which are not contraindicated for PAD). Finally, the international REACH registry also evidenced the under-treatment of PAD patients [57].
In a population-based tele-interview investigation in the US, a representative group of 2,501 adults aged 50 or older were interviewed as to the issues of PAD, risk factors for cardiovascular disease and other underlying cardiovascular disorders [58]. Only 26 % of the subjects in the sample were knowledgeable about PAD, and half of this group was unaware that diabetes mellitus and smoking increase the risk for PAD. Only one out of four respondents knew that PAD is accompanied by increased mortality due to myocardial infarction and stroke. Only 14 % of the interviewees were aware that PAD may become acute and may result in amputation. Respondents who were at the highest risk for PAD due to their risk factor constellation and current underlying disease showed a deficient level of knowledge and/or awareness of PAD. The authors of this study concluded that the public is insufficiently informed about PAD [58].
Encouragingly although there is still under-treatment of PAD, the figures are improving. For example, Sigvant et al showed that among 18,742 revascularized PAD patients in the national Swedvasc registry 2008–2013 antiplatelet therapy, statins, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, and beta-blockers were used by 73 %, 60 %, 57 %, and 49 % at admission for revascularization. Best medical treatment, defined as any antiplatelet or anticoagulant therapy along with statin treatment, was offered to 65 % of patients with intermittent claudication and 45 % of patients with critical limb ischaemia [59].
3 Diagnosis of peripheral arterial disease
3.1 General clinical examination of the limb: Inspection, palpation and auscultation
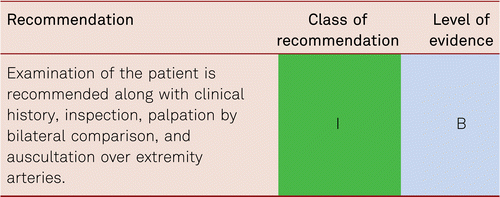
Diagnostic techniques in patients with PAD should be PAD stage- and patient-oriented, targeted and accurate. Furthermore, account should be taken of the risk-benefit ratio and cost effectiveness of each technique. Initially, medical history and thorough clinical examination should be performed with vascular auscultation and palpation.
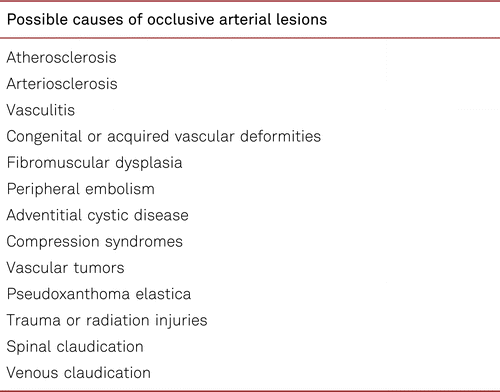
Changes and status of skin, muscular abnormalities, and orthopaedic deformities and the color, hairiness and temperature of the legs and feet should be documented by bilateral comparison. Clinical neurological examinations of the lower limbs are useful especially if there are signs of concomitant neuropathy with changes in foot anatomy and/or skin physiology. This should be recorded and evaluated with a risk score [51]. The differential-diagnostic symptoms of neurological and/or orthopedic disorders should be considered to assess the clinical relevance of possible impaired peripheral circulation. Patients with claudication or critical limb ischemia frequently show concomitant neurological or orthopedic disorders which may confound the diagnosis of PAD. Likewise, other causes should be ruled out in patients who have trophic disorders and ulceration of the lower limbs, excluding such conditions as venous ulceration, vasculitis, summarized in Table 3.1.1.
Pain typically caused by claudication consists of reproducible exercise stress-dependent myalgia which improves rapidly within minutes at rest. Depending on the localization of the vascular lesions, such pain may develop in the gluteal region or the thigh, calf, or foot muscles. Pain affects the ability to walk. Reduced walking performance can be described as a measurement of pain-free maximum walking distance and/or impaired walking speed. Unlike CLI, blood circulation in the affected extremities at rest is still sufficient. In stenosis of the proximal pelvic arteries, pulses are often palpable and Duplex spectra may be triphasic ie normal, at rest.
Pain at rest and/or trophic skin-and tissue- lesions are present in CLI. Pain at rest always affects the part of the limb most remote from the last open vessel, mostly the forefoot. Lowering the leg frequently results in relief from pain symptoms. CLI is generally defined by the loss of balance between arterial perfusion and the metabolic oxygen and nutrient demands of the tissues.
In general, pain in the lower limb or foot can also be caused by a primary or concomitant neuropathy. Table 3.1.2 gives some orientation about the clinical signs of the two entities.
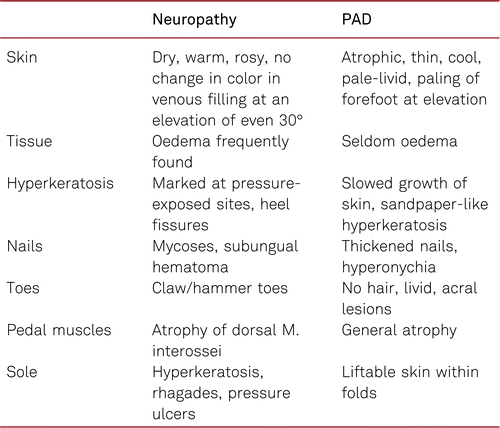
3.1.1 Significance of pulse examination
Pulse examinations at the lower limbs are helpful, yet defective [60]. Pulse palpation alone is insufficient to detect PAD. This disorder is too frequently diagnosed according to poor or absent pedal pulses, as opposed to diagnosis according to typical claudication symptoms [7]. We know the pulse palpation is wrong in over one third of cases due to clinician error, oedema of foot or anatomical variation in vessels. With a sensitivity of 20 %, pulse palpation alone is insufficient to detect PAD and is to be combined with auscultation as basic examination (sensitivity: 75 %; specificity: 40 %) [60]. According to the Basle study, the combination of palpation by bilateral comparison and auscultation, together with a history of claudication, shows an efficiency of detection of 84 % for clinically relevant stenosis [61].
While discomfort due to claudication in the calves is easily identified, exercise stress-dependent pain in the soles or gluteal region – in the presence of occlusions in the lower-leg or pelvic arteries – is frequently diagnosed with more difficulty, by purely clinical examination.
This applies specifically to patients with diabetes and, as shown in Table 3.1.2, assists in differentiating between primarily neuropathic and ischemic changes/lesions [62]. Diabetic autonomic and symmetrically sensory and motoric polyneuropathy is especially frequent in patients with diabetes with PAD, and special protective footwear is recommended in the presence of severely impaired perfusion or typical signs and changes of neuropathy. Regular professional foot examinations carried out by physicians, podiatrists or trained patients themselves, may increase awareness and are invaluable in terms of skin damage prevention [63, 64].
3.2 Significance of the ankle-brachial index
Alongside inspection, palpation and auscultation basic examinations of vascular status include Doppler ultrasonography of occlusion pressure in the dorsal pedal and posterior tibial arteries and, as appropriate, the peroneal artery, in recumbent patients and the calculation of an Ankle-Brachial Index (ABI) [5, 45].
The subjects should not have over-exercised before their examination (e.g. by bicycling or running longer stretches). After approximately 10 minutes at rest in supine position, the patients undergo two systolic blood pressure measurements, the first along the brachial artery according to Riva-Rocci [65]. The mean value of both measurements is to be estimated (exception: the respectively higher pressure value is used in pressure differences ≥ 10 mmHg). 10 to 12 cm blood pressure cuffs are inflated above the ankle and systolic values measured and noted along the posterior and anterior tibial arteries with a Doppler probe (8 to 10 MHz) with the higher pressure selected for calculation of the ABI.
In addition to Doppler ultrasound measurements, several other non-invasive techniques have been described for ABI measurements, primarily oscillometric methods. The American Heart Association Scientific Statement on the Measurement and Interpretation of the Ankle-Brachial Index contains a detailed description of these methods and an analysis of validation studies [66]. In summary, the ABI measured by many of these alternative methods correlates well with Doppler-measured ABI scores in healthy and mildly affected subjects. However, correlations are poor when the ABI measured by Doppler is in the low range. Furthermore, reproducibility is poorer and intra-observer variability is higher with these methods as compared to the Doppler method. Therefore, the Doppler ultrasound method should in general be used to measure the ABI.
The ABI threshold score was derived from comprehensive epidemiological studies. The value is defined as 0.9 in the guidelines issued by the European Society of Cardiology (ESC) [28], the American College of Cardiology/American Heart Association (ACC/AHA) [67], the National Institute for Health and Care Excellence (NICE) [68], and the Transatlantic Inter-Society Consensus (TASC) II [7]. The lower the score, the stronger the atherosclerotic changes in the leg, and blood-flow obstruction is significant (Table 3.2). It should be noted, however, that claudication discomfort may develop with quite divergent ABI values in different individuals. Also, with medial sclerosis ABI may be falsely normal or increased.

Having critical ischemia is considered to be a crucial prognostic factor for the healing of peripheral lesions and has been described, alongside clinical symptoms, by an ABI score of < 0.5 and a pulsatility index of ≤ 1.2 with a sensitivity and specificity of 36 % and 86 %, resp. 87 % and 67 % [7].
3.2.1 Calculation of the ABI
The ABI can be determined by a physician, vascular technologist, trained podiatrist or nursing staff. A comprehensive systematic comparison of examination results obtained by angiologists, family physicians, and assistants in healthy subjects demonstrated no differences between the ABI values measured by three different professional groups in individual patients and a low, approx. 8 % level of variance between repetition measurements [69]. In spite of the high level of accuracy, it should be noted that, due to measurement error, confirmatory repeat measurements should thus be carried out in patients whose ABI values approximate to the threshold value of 0.9.
The ABI is commonly calculated either by dividing, first, the highest of the obtained pressure values in the foot (posterior tibial artery [PTA], dorsalis pedis [DP]) by the highest or medium arm pressure values or, second, the highest of the obtained calf pressures in the foot by the highest or medium arm pressures. There is consensus that the mean systolic blood pressure (SBP) values of both arms should be taken as denominator unless the systolic blood pressure difference exceeds 10 mmHg, in which case the highest SBP value should be taken as denominator and a subclavian artery stenosis should be ruled out at the side with the lower pressure [66]
The first method (taking the lowest value of the foot) may be used to determine the presence of arterial disease and thus to predict cardiovascular morbidity and mortality with a high degree of sensitivity and specificity. The consequence of this prediction, however, remains unclear, in particular in asymptomatic patients. To date, there is no evidence that the prophylactic treatment of such patients with statins or platelet inhibitors would lower cardiovascular morbidity and mortality, although intellectually this makes sense [17].
The second method (taking the highest value of the foot) is used to determine the presence of ischemic disease and thus to diagnose PAD on a functional level. Normal ABI measures at rest do not rule out PAD in symptomatic patients. In such subjects, it is strongly recommended that a treadmill test with ABI measurements be carried out immediately after reaching maximum walking distance. Furthermore, medial sclerosis may obscure the presence of peripheral arterial disease, which means that the ABI is in normal or supernormal range in the presence of clinically relevant PAD. Measurements of toe pressure and calculation of a Toe Brachial index (TBI) instead of an ABI are recommended especially in these patients and in patients with diabetes. TBI result accuracy is enhanced by pre-heating of the foot prior to measurement.
3.2.2 Exercise tests to objectify claudication
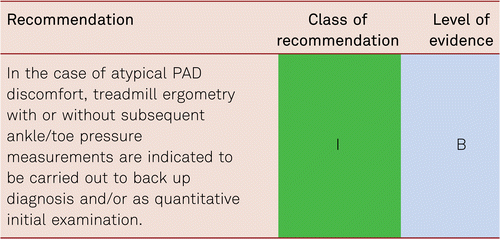
It should be noted that ABI values of > 0.9 can be found in well collateralised, proximal high-grade stenoses or occlusions, or with haemodynamically borderline stenoses. De-masking is accomplished by measuring the ABI after exercise. Thus, in the presence of non-conclusive ABI findings, an additional ABI measurement at rest immediately after physical exercise by repetitive tiptoe standing, treadmill or ergometric stress increases the sensitivity of detecting PAD at rest by 10 %. Detection of a monophasic frequency spectrum after stress also improves the sensitivity for PAD masked at rest [70] (See also Chapter 3.3.2).
The technique is as follows: The ABI is measured at rest and subsequently walking performance (e.g. on a treadmill at 3.2 km/h and 12 % incline, i.e. 100 W load) is measured. Pain-free and maximum walking distances are documented, as well as walking time and ankle pressure after stress. An ABI decrease of 20 % confirms the diagnosis [70].
In the absence of a treadmill, the exercise may be carried out by supervised rapid walking along a defined corridor stretch. Patients incapable of treadmill examinations or rapid level walking can be examined by active plantar flexion. Findings are seen to correlate very well with those obtained in treadmill ergometry [71].
The success of claudication therapy can be objectively quantified with exercise testing. Initial values of pain-free and maximum walking distances or absolute walking time serve as comparative parameters of PAD patients’ walking performance. Objectified stress in terms of walking time and distance as well as patient-based validated disorder-specific questionnaires (e.g. Medical Outcome Short Form SF-36, Walking Impairment Questionnaire [WIQ]) can be applied to evaluate treatment [72].
Exercise testing also plays an important role in the differential diagnosis of claudication. The differential diagnoses of PAD are listed in Table 3.2.2.
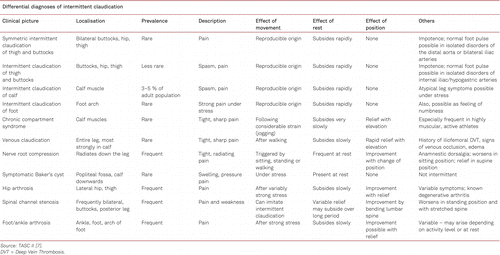
Besides the PAD with different complaints, depending on the localization of the arterial narrowing or occlusion, there are some pathologies which cause comparable symptoms but have another underlying cause. For instance, spinal claudication will not lead to a pressure decrease after exercise.
3.2.3 The ABI in medial calcific sclerosis
In patients with diabetes, the ABI is unhelpful in PAD diagnosis in 10 to 30 % due to Mönckeberg’s arteriosclerosis (> 1.3 high values in a situation of true ischemia). Apart from an ABI value ≥ 1.3, a (pseudo) normal ABI score with flattened Doppler pulse curves (acoustic or graphic reduction of pulsatility) indicates the presence of medial calcific sclerosis with relevant stenoses [66].
The association between the ABI and cardiovascular mortality/morbidity is different between patients with diabetes and patients without diabetes. While patients without diabetes feature a linear correlation between increasing risk and decreasing ABI values, the correlation in patients with diabetes corresponds to a U-shaped curve: not only ABI scores ≤ 0.9 but also ≥ 1.3 are associated with increased cardiovascular risk [73].
Apart from medial calcific sclerosis, falsely high pressure values in peripheral ankle pressure measurements are also found in peripheral edema or cases in which perfusion of the ankle arteries is exclusively fed by an unobstructed fibular artery. To rule out medial calcific sclerosis in the arm arteries, a permanently elevated systemic blood pressure ≥ 250 mmHg necessitates a duplex examination or alternately an X-ray-radiography of the upper arm.
Another equally sensitive method in medial calcific sclerosis, the pole test, uses hydrostatically assessed pressure values at the hallux or the dorsal pedal artery as measures. Passive leg elevation is applied (and the distance in cm from bottom line, in which the Doppler signal vanishes corresponds to the arterial perfusion pressure in the extremity; 1 cmH2O corresponds to 0.74 mmHg) instead of the sphygmomanometer technique [74].
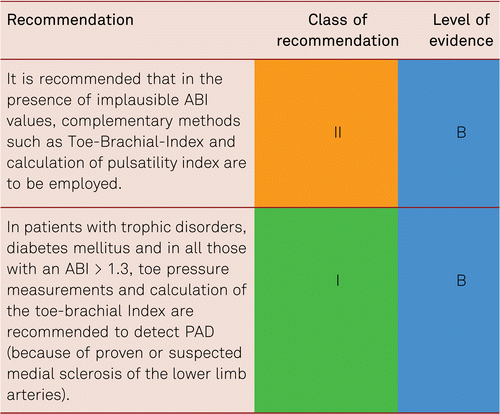
3.3 Complementary methods of measurement in implausible ABI scores
3.3.1 Toe-brachial index
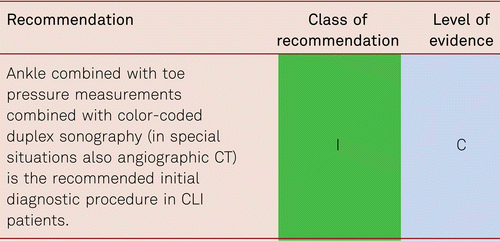
Medial sclerosis occurs in patients with diabetes mellitus, severe renal insufficiency and long-term immunosuppressive therapy. Since medial calcific sclerosis affects the digital arteries less than the lower-leg arteries, assessment of hallux pressure with readings ≤ 30 mmHg offers an additional sign for the presence of CLI. Toe pressure is approximately 30 mmHg below systolic ankle pressure, and the pathological Toe-Brachial Index (TBI) is 0.7 and less. Measuring the pressure in the big toe mainly measures perfusion via the posterior tibial artery. Additional toe measurements are recommended, as the perfusion in the lateral half of the forefoot is provided by the anterior tibial artery and cannot be evaluated by a great toe measurement.
Contrary to the ABI, the TBI shows a linear association with cardiovascular events and thus differs from the U-shaped curve of the ABI in medial calcific sclerosis. There is one prospective comparative study in diabetic and non-diabetic patients [75]. Therefore, it should be used in the presence of implausible ABI readings. Regardless of coincidental diabetes mellitus, the ABI and the TBI are strongly positively correlated in the presence of ABI values ≤ 0.9 and ≤ 1.4 [75, 76].
While the ABI threshold of ≤0.9 as a cardiovascular risk indicator has been validated in many cases, the TBI threshold of 0.7 is scientifically less well proven and requires further validation [77].
3.3.2 Doppler frequency spectrum
Evaluation of the acoustically or graphically documented Doppler frequency spectrum results in valuable additional parameters in the diagnosis of PAD. In one investigation, one third of the patients with occlusions of the lower-leg arteries showed normal ABI values at rest and under stress. Relevant PAD was detected by applying the criterion of “monophasic Doppler frequency curve” [6]. The classic monophasic Doppler frequency curve at the common femoral artery showed a highly positive predictive value of 92 % in patients with significant aortoiliac lesions [78].
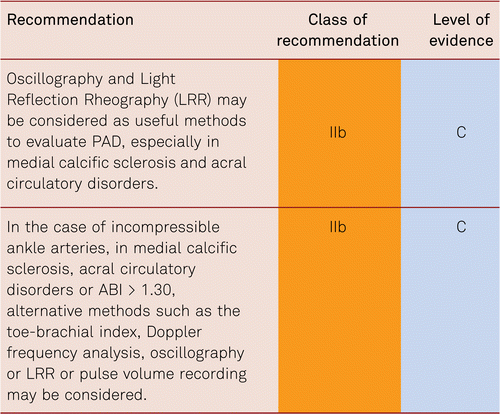
3.3.3 Oscillography
As additional non-invasive methods, oscillography and light reflection rheography (LRR) may prove helpful in specific questions (e.g. presence of medial calcific sclerosis). The advantage of mechanical and electronic oscillography is its quick and simple feasibility and its possibility to identify the approximate level of stenosis or occlusion [65].
LRR of the digital arteries is helpful in acral perfusion examination, also for bilateral comparison. The form of the pulse curves facilitates rapid diagnosis of the presence of peripheral blood-flow disorders and offers a good impression about the severity of the perfusion disorder. However, no sufficient evidence for these two methods has emerged from recent studies.
3.3.4 Transcutaneous oxygen pressure measurement
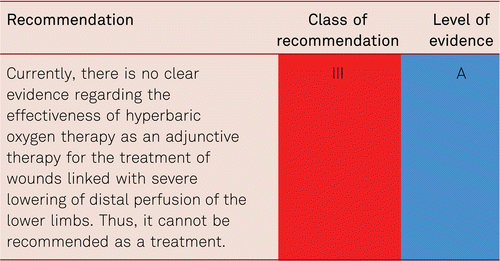
Assessment of transcutaneous partial pressure of oxygen (TcPO2) can additionally be used especially to estimate the risk and or level of amputation in CLI. CLI is defined as a TcPO2 value < 30 mmHg in supine patients, yet it is dependent on several influential variables (skin texture, anemia, blood oxygenation, i.a.) and relevant data are deficient. A TcPO2 value < 40 mmHg is associated with an increased complication rate following amputations, and a value < 30mmHg is an independent predictor of wound-healing disorders (OR 3.21, 95 % CI 1.07–9.69) [79]. The amputation risk in the presence of TcPO2values < 10 mmHg is 70 % [78].
The certainty of detecting CLI is improved by changing leg positions (from a lying to a sitting position) without an increase in TcPO2 values [80]. Reactive hyperemia can be used to assess skin perfusion by changing probe temperature (probe heating to 37°C or 44°C) and oxygen inhalation. To increase validity, it is recommended to carry out measurements over at least 3 different positions on the involved extremity.
Due to the limited sensitivity and specificity of this method, a combination of various analytical procedures (clinical, Doppler methods, capillary microscopy, TcPO2measurements) may be used to quantify circulation and assess the chances of healing, though little evidence exists for their use.
3.5 Non-invasive diagnostic imaging procedures
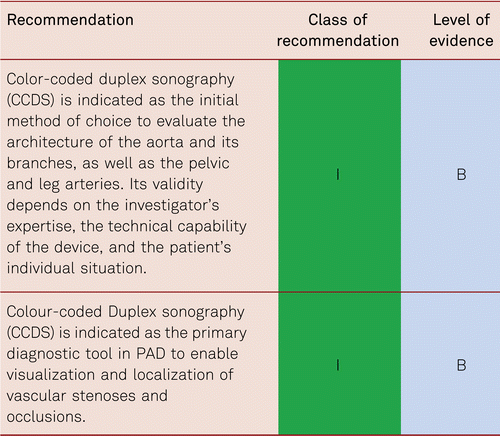
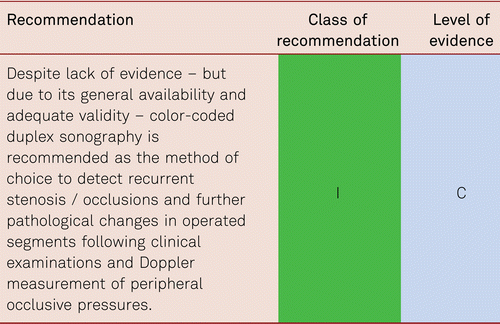
3.5.1 Colour-coded duplex sonography (CCDS)
Vascular duplex ultrasonography has become the single innovative diagnostic method in all areas of everyday vascular medicine. For the stepwise diagnosis of PAD, it occupies a key role in treatment planning prior to invasive procedures. It is the diagnostic imaging method of choice for both arteriosclerotic and non-arteriosclerotic vascular disorders.
CCDS is a non-invasive method of investigation, which facilitates successful surgical or endovascular recanalization in claudication as well as in CLI. This was demonstrated by a retrospective evaluation of intraoperative and/or peri-interventional digital subtraction angiography (DSA) images of 4,783 patients with infrainguinal Transatlantic Inter-Society Consensus (TASC) C and D lesions. With a sensitivity of 97 % and a specificity of 98 %, CCDS as sole diagnostic method was successfully used in treatment planning and decision-making for bypass surgery or endovascular treatment [81]. With CCDS, important differential diagnoses can be identified, including vasculitis, muscular compression syndrome, aneurysmal vascular occlusion, and rare cystic adventitial degeneration.
Duplex sonography is widely available, non-invasive, reproducible and biologically inert. Applied by experienced investigators, it shows high levels of sensitivity and specificity [82, 83] and, based on reliable findings, facilitates the safe planning of necessary treatment steps (conservative treatment, catheter intervention, bypass surgery) [81]. It provides morphological imaging of vascular walls, perivascular tissue or details of early atherosclerotic change by capturing intima-media thickness (IMT), and thus delivers a valuable parameter for clinical studies [64, 84].
Color flow imaging facilitates full understanding of the hemodynamics of stenoses and occlusions [85], as well as offering valuable and valid direct and indirect flow parameters [78]. The disadvantages of this method include a high level of investigator dependency, artifact interference (medial calcific sclerosis, calcifications, electromagnetic fields), and elaborate documentation. Further disadvantages include difficulty in imaging aorto-iliac vessels in patients with significantly elevated body mass index (BMI).
The use of contrast agents suitable for ultrasound may serve to further enhance the validity of duplex sonography findings. Routine contrast application, however, is not required.
3.5.2 Computerized tomographic angiography (CTA)
Due to widely available modern multi-slice CT machines, CTA has been established as a valid method of measurement with high levels of sensitivity and specificity in vascular disorders [86, 87]. Drawing on 20 studies in 957 patients with CI or CLI (at least 10 participants per study), a systematic review found a sensitivity of 95 % and specificity of 96 % for CTA to detect at least a 50 % stenosis (measured by DSA). CTA correctly diagnosed vascular occlusions in 94 %, and at least a 50 % stenosis in 87 %, and the absence of significant stenosis in 96 %. However, the methodical quality of the included studies was limited: Only 2.2 % of a total of 909 studies with data comparing CTA and DSA were eligible for the meta-analysis [88].
The method produces high-quality, multiplanar and three-dimensional images of the aortoiliac, femoropopliteal, and crural vascular system and its surrounding anatomical structures. Centerline reconstructions facilitate exact computations of interventional and reconstructive surgical procedures and are indispensable for measuring endografts in aortoiliac vessels. The advantages of the method consist of a very short time of investigation, the detectability of treatment-relevant comorbidities that may imitate PAD symptoms, a spatial resolution within the submillimeter range, and an imaging potential that is surgically important in anatomic-topographic terms.
The disadvantages of CTA include exposure to radiation, the need for iodine-containing contrasts (with a contrast volume of approx. 100 ml per examination), the overestimation of stenosis grades in the presence of small-caliber vessels with calcified stenosis i.e. crural vessels (although these can be improved by use of dual-source CT), and the effort expended in image post-processing depending on the age of the machine.
3.5.3 Magnetic resonance angiography (MRA)
MR angiography is a non-invasive, investigator-independent imaging methods. High-quality three-dimensional vascular reconstructions can be produced with high levels of sensitivity and specificity by applying common MR tomography, surface coils and three-dimensional gradient echo sequences [82]. Data from a small meta-analysis have shown a pooled sensitivity of 86 % and specificity of 93 % compared to the gold standard DSA for the infragenicular region [89]. There is an urgent need for more comprehensive studies, as the absence of radiation exposure with MRA is a significant benefit over CTA.
Such techniques as time-of-flight angiography and phase-contrast angiography, as used in cerebral vascular diagnosis, are unsuitable for PAD diagnosis. Therefore, contrast-enhanced magnetic resonance angiography (CEMRA) is considered the standard in pelvis-leg vessel imaging. Like DSA, this method provides initial non-contrast examinations of the aortoiliac, femoral, and crural regions. After determining the ideal bolus time (test bolus), the measurements are repeated after contrast administration and subtracted from one another. Resulting subtraction images are calculated as maximum intensity projections and require no post-processing. By making use of first-pass effects, it is possible to obtain images that are free from super-positions and high in contrast. As in CTA, the examination is standardized and completed, together with reconstructions, within less than 30 minutes.
The advantages of MRA include a rapid and simple acquisition of valid and clear angiographic images without potentially nephrotoxic contrast media (nevertheless older linear MR-contrast media are contraindicated in severely impaired renal function because of the risk of nephrogenic systemic fibrosis) and without exposure to radiation. The disadvantages are MR contraindications (magnetic metal implants, cardiac pacemakers, severe renal failure) and limited image quality due to movement artifacts. Overestimations of intermediate stenosis grading in severe stenoses may occur, especially in small-caliber vessels (susceptibility artifacts) and aortic side branches.
The contrast doses applied in CEMRA are frequently higher than in other indications. For instance, 0.1 mmol/kg BW are recommended for central nervous system and liver applications, and the 2- to 3-fold quantity (0.3 mmol/kg BW) in CEMRA, an examination that thus applies relatively high contrast doses. Gadolinium-based contrast agents have a 6- to 8-fold lesser rate of allergic side effects (1 %) and are not per se nephrotoxic. However, linear gadolinium-based contrast agents had a serious side effect, nephrogenic systemic fibrosis (NSF), which is specific in comparison to other contrast media. Recently, also new contrast-free MR based methodologies have become available in specialized centers.
3.5.3.1 Nephrogenic systemic fibrosis (NSF)
In 2006, NSF was suspected to be associated with the administration of linear gadolinium-based contrasts for the first time. NSF has so far only been observed in patients with severe renal failure (GFR < 30 ml/min/1.73 m2), those with acute renal insufficiency due to hepatorenal syndrome, or perioperatively in liver transplantations. This applies especially to dialysis patients who have been examined with linear gadolinium.
The disorder led to skin sclerosis with swelling and contractures and other systemic organ involvements. NSF became progressive in 5 % of patients. In terms of etiology, it is considered to represent a multifactorial process. Predisposing factors included surgical operations, vascular injuries, high doses of erythropoietin, and high levels of serum phosphate. No precise frequency rates are known. The Yale NSF Registry reported 380 cases by 2013 (www.icnfdr.org), although causalities have not proved definitive in all cases [90]. No causal connection with the emergence of NSF has so far been reported following the administration of macrocyclic gadolinium-based contrasts.
To prevent gadolinium-induced NSF, contrast use should be critically assessed in patients requiring dialysis and those with severe renal failure and a GFR of < 30 ml/min/1.73 m2, and other imaging methods should be considered. However, some macrocyclic MR-contrasts are also approved for use in patients with manifest renal failure. The ionic linear chelate gadodiamide should not be used if possible. In cases in which it must be used, the quantity is to be dosed as small as possible and following contrast examination, a timely hemodialysis should be carried out in patients with terminal renal failure. No further gadolinium-based contrast agent is to be used in future if NSF is clinically suspected.
3.6 Intra-arterial angiography (digital subtraction angiography, DSA)
Intra-arterial DSA is considered to be the gold standard in terms of the accuracy and clarity of vascular imaging. However intra-arterial angiography as a purely diagnostic procedure is increasingly superseded by highly sensitive and specific non-invasive procedures, such as CCDU, MRA and as appropriate, CTA.
The advantages of intra-arterial DSA consist of good documentation, high levels of experience as an established procedure, and the opportunity to combine diagnostics and interventions in one single session. DSA is substantially more precise than any other imaging procedure, particularly in evaluating in-stent stenoses. The combination of ultrasound Doppler frequency spectrum analysis and DSA ensures an optimal validity.
Disadvantages result from the invasiveness of this analytical method. Potential complications include hematoma, false aneurysm, bleeding, arteriovenous fistula and contrast-related complications (contrast-induced nephropathy, allergic contrast reaction, contrast-induced hyperthyroidism), which influence subsequent patient management in 0.7 % of cases and are associated with a mortality rate of 0.16 % [7]. Complication rates in intra-arterial angiography depend on comorbidities (heart failure, renal failure) and the presence of other risk factors (advanced age, diabetes etc.) and amounts to 0.5 to 1 %.
3.6.1 Risks of iodine-containing contrast agents and prophylactic measures
Contrast-induced nephropathy (CIN) is defined as impairment of renal function secondary to the use of contrast medium for radiological procedures. It is measured as either a 25 % increase in serum creatinine from baseline or 0.5 mg/dL (44 μmol/L) increase in absolute value, on condition that there is a temporal connection with intravenous or intra-arterial contrast administration and there are no other causes. The increase may be delayed by up to 7 days after contrast administration.
The frequency of CIN varies depending on its definition, concomitant risk factors, the kind and dose of contrast agent, and its route of application [91]. It occurs in 1 to 6 % in the normal population and reaches its lowest levels where there is normal renal function and no other risk factors, after intravenous and intra-arterial contrast administration (1 % and 3 %, resp.). In the presence of risk factors, the incidence increases to 14 to 50 %. The frequency of CIN with at risk patients requiring dialysis treatment has been reported to be 0.8 % [92–94]. Patient-specific risk factors include preexisting chronic renal function disorders, diabetes mellitus, age above 75 years, manifest heart failure, dehydration and hypovolaemia, nephritic syndrome and multiple myeloma. The necessity of routine assessments of serum creatinine is subject to debate. Examination-related risk factors include intra-arterial delivery, high contrast dose, highly viscous and highly osmolar contrast agents, and multiple contrast administration within 48 hours. Surveys have shown a 13 to 20 % rate of such assessments prior to intravenous contrast administration [95].
Recommendations have assumed that the risk for CIN increases above a serum creatinine level of > 1.4 mg/dl. This value may be misleading in older patients and those showing little muscle mass, as it corresponds to an approx. 50 % reduction in renal function in normal-weight subjects. Therefore, it is more robust to estimate the glomerular filtration rate (GFR), rather than to exclusively use screening parameters.
Nephrology Scientific Societies thus recommend grading chronic renal failure according to the GFR. Moderate chronic renal failure with an increased risk of CIN is thought to occur at a GFR of < 60 ml/min [96]. In the presence of moderate renal failure with a GFR of < 60 ml/min prior to contrast administration, a second reading of serum creatinine and the GFR should be done 24 to 72 hours after contrast administration in order to establish or rule out CIN.
Metformin may cause lactic acidosis in connection with CIN. If safe, metformin-containing oral antidiabetic drugs should be discontinued 2 days before and withheld for 2 days after planned contrast administration. In known chronic renal failure, CIN (GFR < 40 ml/min) is to be ruled out prior to restarting the drug because of already established concerns about metformin use in cases where the GFR is < 50 ml/min.
3.6.2 Prophylaxis against CIN
Sufficient intravenous hydration (10 ml/kg body weight) depending on comorbidities (e.g. heart failure) is recommended. Fluid substitution in patients at risk should be carried out 12 hours before and up to 12 hours after contrast administration. Volume administration is to be modified in the presence of heart failure. Additional protective measures include the discontinuation of non-steroidal anti-inflammatory drugs (especially diclofenac), discontinuation of diuretics (as far as possible), reduction of contrast administration to the absolute necessary minimum, and warming of contrast agents to reduce viscosity.
In the past acetylcysteine was recommended as prophylaxis. However, no scientific evidence of effectiveness has so far been produced for this recommendation [97] and the latest Preserve Trial [98] showed no advantage of bicarbonate and/or acetylcysteine over sodium chloride infusion alone.
The value of theophylline, aminophylline and ascorbic acid administration has not been verified.
Sufficient data do not exist to support post-treatment prophylactic hemodialysis in an attempt to rapidly eliminate contrast media.
3.6.3 Iodine-induced hyperthyroidism
Assessment of basal thyroid-stimulating hormone (TSH) values serves to exclude a diagnosis of hyperthyroidism. Patients with severe concomitant disorders and a poor general condition may present with suppressed TSH due to non-thyroidal causes. Subjects with latent hyperthyreosis and/or adenomatous goiter, as well as scintigraphically evidenced over activity are at risk.
The recommendation for prevention of iodine-induced hyperthyreosis is 900 mg sodium perchlorate daily. In cases of manifest hyperthyroidism, optionally 10 to 20 mg daily methimazole can be given no later than 2 to 4 hours before contrast administration and then after contrast exposure for 14 days. Should suppression prove not to be feasible, iodine-containing contrast agents should not be used in manifest hyperthyreosis or latent hyperthyreosis and/or over-activity.
Sodium perchlorate given at 900 mg daily is indicated in manifest hyperthyroidism. In such cases 40 to 80 mg/d methimazole are also to be administered for 14 days. Adjusted doses of methimazole are to be given thereafter. In manifest iodine-induced hyperthyroidism, methimazole is to be dosed at 40 to 120 mg/d over weeks to counteract iodine. A combination with sodium perchlorate serves to accelerate normal thyroid function [99].
3.6.4 Carbon dioxide angiography
In patients with renal failure, carbon dioxide (CO2) angiography is an alternative to intra-arterial angiography using nephrotoxic contrast agents. Sufficient imaging of the pelvic and thigh vasculature is feasible up to the popliteal artery and the proximal calf arteries and can help to prevent CIN during peripheral interventions [100]. Endovascular treatment supported by reliable documentation is also possible with images of the calf vessels and (if necessary and possible) a few milliliters of conventional iodine-containing contrast.
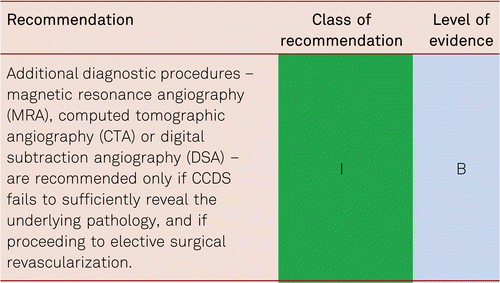
3.7 Choice of imaging diagnostic procedures
The on-site availability of modalities and investigator expertise is to be considered in choosing whether to select CCDS, CTA, MRA, or intra-arterial angiography. However, in most units this means that CCDS should be the first choice, however if this technique does not reveal the underlying problem or the images are limited, then other modalities should be chosen. Likewise, patients’ individual conditions are to be considered taking into account concomitant disorders (e.g. renal failure, thyroid disorders, heart failure, and cardiac pacemakers). Table 3.7 summarizes the significance of various imaging procedures.
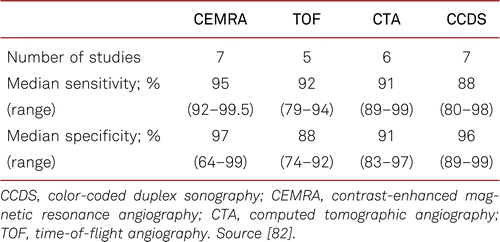
The most important reason for imaging is a clinical situation in need of treatment. The objective of imaging is to evidence and further characterise the vascular lesions with PAD, which then are preferably revascularised by interventional or surgical treatment as required. Furthermore, other differential diagnostic causes of vascular lesions (e.g. aneurysm) or discomfort (e.g. compression syndromes) can be excluded by imaging.
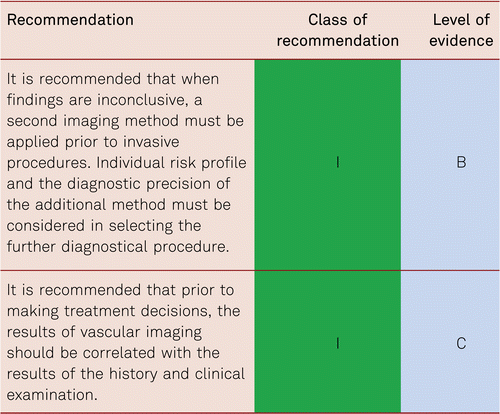
In summary, imaging diagnostics are necessary for patients with PAD who are suitable for invasive treatment. Prior to this, the disorders should be detected with non-invasive hemodynamic diagnostic methods and assessed in connection with history and clinical symptoms [101].
4 Therapy for peripheral arterial disease – introduction
This chapter introduces the concepts for conservative medical treatment for chronic PAD in all stages: the asymptomatic stage (PAD I according to Fontaine, Rutherford 0), intermittent claudication (Stage II according to Fontaine, Rutherford 1–3), and critical limb ischemia (Stages III and IV according to Fontaine, Rutherford 4–6). Medical treatment recommendations in terms of peri- and post-interventional platelet inhibition and anticoagulation are outlined in the section on peri-interventional management.
It must be remembered that alongside structured exercise and medical treatments, endovascular and surgical therapies are integral to patients with PAD.
Patients’ treatment compliance is often reduced because of lack of knowledge, thus impeding conservative treatment, in particular. Despite structured exercise being a recognized constituent of optimal vascular treatment, it is efficiently and regularly practiced by very few patients. Further, warning signals of worsening symptomatic PAD are often misinterpreted or ignored by both the patient and the uninformed clinician.
4.1 Principles of PAD therapy
The number of patients with PAD is continuously increasing due to the aging population and growing number of patients with diabetes. By 2020, the workload for vascular medicine will have increased by more than 40 % compared to the beginning of the millennium [102].
The cornerstone of PAD therapy is twofold: reduction of cardiovascular risk factors and concomitant disorders, especially CAD and cerebrovascular diseases, and secondly, the improvement of peripheral blood flow in symptomatic patients. Depending on the clinical stage of disease, the emphasis on specific treatment modalities change. Thus, for asymptomatic and long-distance claudication patient’s emphasis is put on the reduction of cardiovascular risk (stage I according to Fontaine, Rutherford 0), for short distance claudication patients, the symptomatic improvement of pain-free and maximum walking distances, the preservation of mobility and thus quality-of-life improvement is important along with CV risk management (stage II according to Fontaine, Rutherford 1-3). Limb preservation is critical in more severe stages, but vascular risk factor modification must not be forgotten (stages III and IV according to Fontaine, Rutherford 4-6, CLI) [7, 28, 67].
4.1.1 Stage-adapted approach
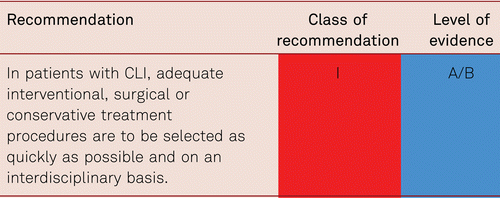
Arterial reconstruction in PAD is a symptomatic treatment and does not resolve the underlying problem of progressive atherosclerosis. Endovascular or surgical interventions are carried out depending on clinical symptoms, localization, and pathology of disease, risk-benefit ratios, and the patients’ individual treatment requests.
There is sufficient available data regarding endovascular and surgical arterial reconstructions to quantify their benefits in appropriate indications, as well as the short- and long-term risks associated with such invasive interventions [48, 103, 104]. These results emphasize the need for interdisciplinary treatment planning prior to vascular reconstructions (also see the section on interventional treatment of PAD and surgical treatment).
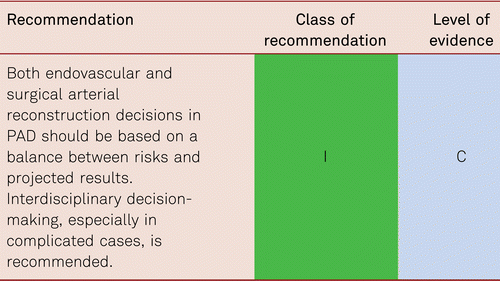
4.1.2 Vascular surgery versus interventional procedures
Open vascular surgery and endovascular interventional treatment for PAD are mutually complementary treatment options and should be allocated appropriately to the right specialist in vascular centers. Appropriate therapeutic procedures can be selected within an interdisciplinary framework while also considering patients’ requests. Many interventionists and vascular surgeons offer joint hybrid interventions that combine surgical and interventional measures.
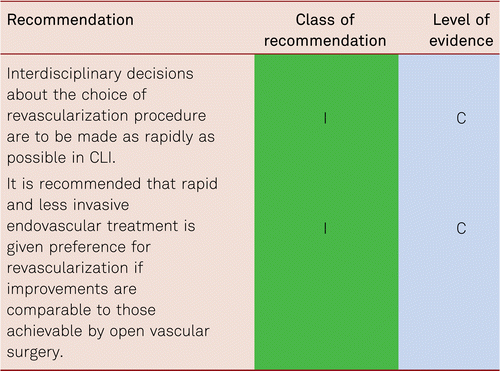
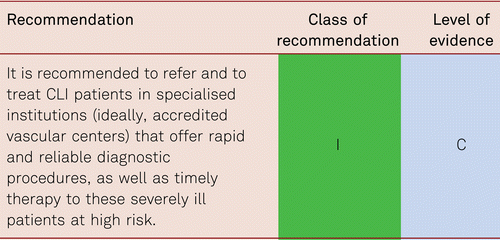
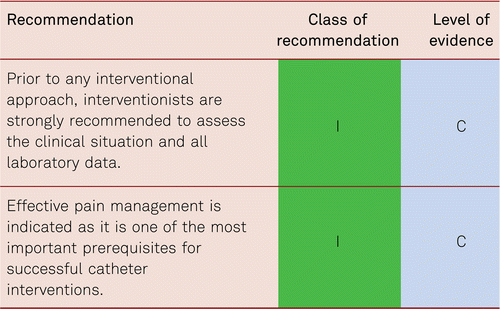
Patients qualifying for vascular interventions often have multiple comorbidities. Surgery as well as catheter intervention thus becomes more difficult and risky with more limited prognosis [105]. As patients increasingly present with more severe concomitant disorders, the rates of perioperative mortality and morbidity is at risk of rising [106]. Minimally invasive, secure, and rapid interventional approaches are, therefore, preferred especially in the CLI patient with multiple comorbidities. These should be interdisciplinary in terms of deciding management pathway.
4.1.3 Procedures in diabetes mellitus
Practically none of the therapeutic studies carried out so far (surgical and interventional revascularization, medical treatment) have separated patients with and without diabetes. Patients with diabetes account for the majority of subjects with progressive vascular disease and CLI, who frequently experience a multiple-stage process and very often severe and diffuse disease in the lower-leg arteries. Frequently, pedal flow is still preserved [107]. However, in these mixed studies treatment options for patients with diabetes are identical to those obtained in patients without diabetes.
Lack of pain perception in diabetic polyneuropathy frequently masks progressive PAD, and in such cases, there is a high risk of neuro-ischemic foot syndrome. For this reason, arterial revascularization is often the first choice therapy in this high-risk group in the presence of hemodynamically relevant vascular lesions.
Another group, the patients with rheumatoid arthritis and connective tissue disorders often present with vascular changes that are comparable to, or even more severe, than those seen in patients with diabetes. This patient group with diffusely affected vessels and often very high-grade focal calcifications represents a special therapeutic challenge. Where inflammation is present e.g. vasculitis, endovascular intervention should not be attempted, and these patients should be initially treated with anti-inflammatory/immunosuppressant drugs, and intervention should only be considered if a relevant stenosis remains present after inflammation resolution.
4.1.4 Procedures in claudication
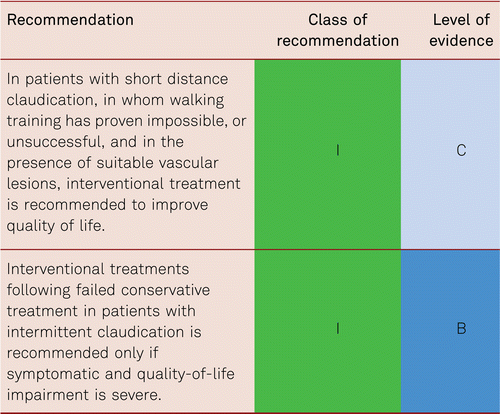
In intermittent claudication, the criteria for vascular surgery and angioplasty should be more strictly applied than in CLI, as in a long-term prospective study, the primary treatment results are no better than with purely conservative treatment. Invasive procedures do not positively affect mortality and leg-preservation rates and/or the patency of leg arteries in long-term observation [103]. The important criteria for treatment in intermittent claudication are the patients’ quality of life and reduction of vascular risk.
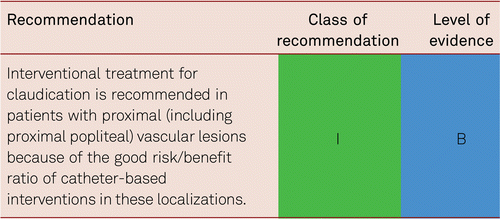
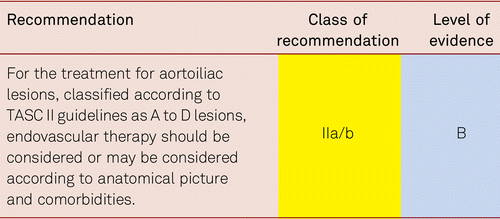
In suitable localizations (proximal lesion) and morphology, endovascular treatment for claudication can be considered [7]. Structured exercise should additionally be offered. The same applies to the surgical removal of severely stenosed lesions of the common femoral artery, which may be indicated in the presence of significant patient suffering.
4.1.5 Procedures in CLI
The primary treatment objectives in CLI are pain relief, healing of trophic disorders and ulceration, avoidance of amputation, improvement of limb function and walking performance, quality-of-life improvement in the short term, and improved survival in the medium term.
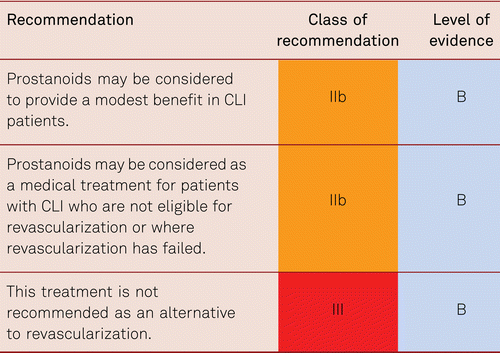
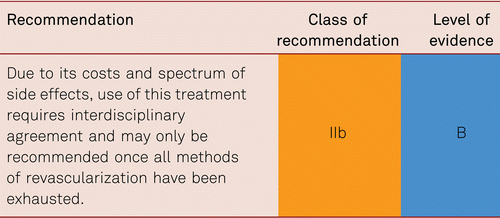
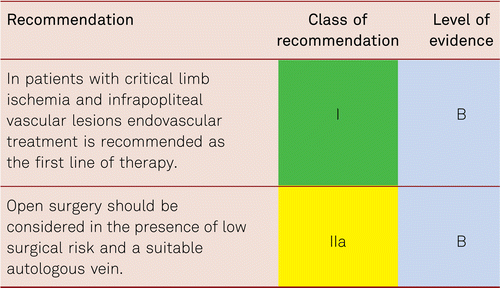
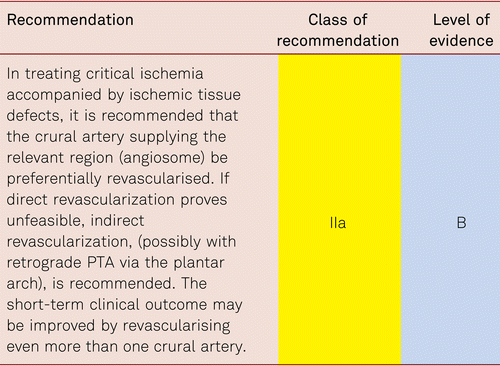
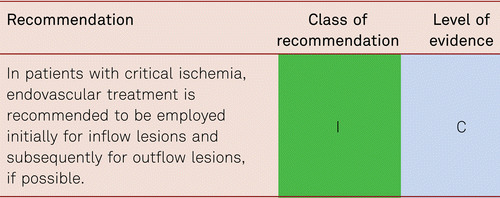
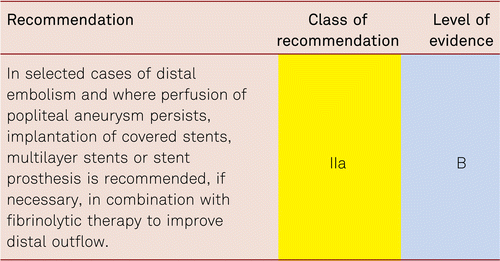
The preferred treatment option in CLI is revascularization [48].
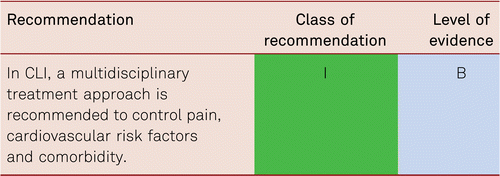
Therapeutic approaches also include analgesic administration and other medical strategies for pain relief, infection treatment and the optimization of cardiac and pulmonary function, if required. Monitoring of and treatment for cardiovascular risk factors is as necessary in patients with CLI as in all other patients with PAD [7].
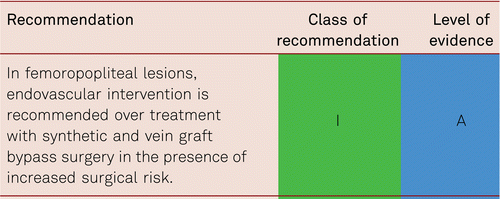
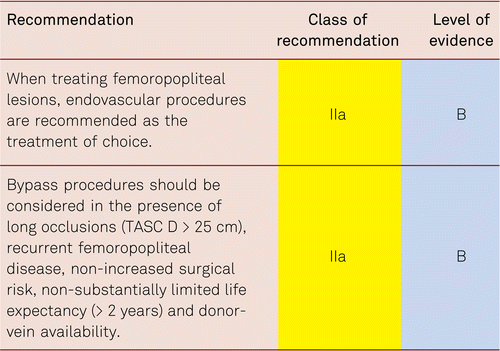
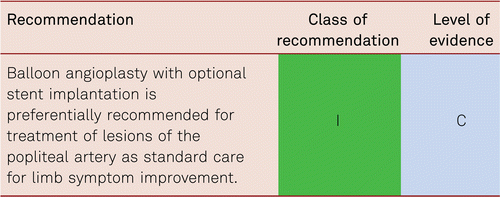
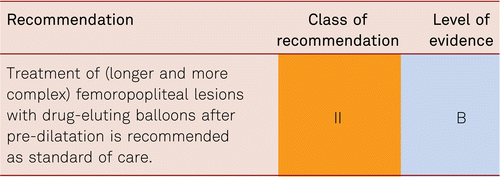
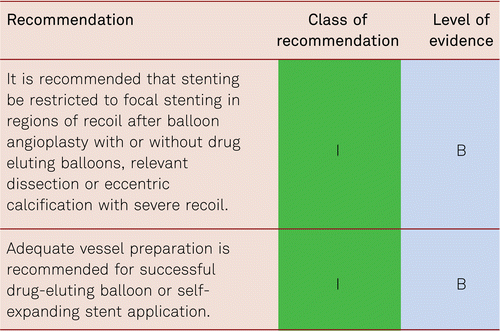
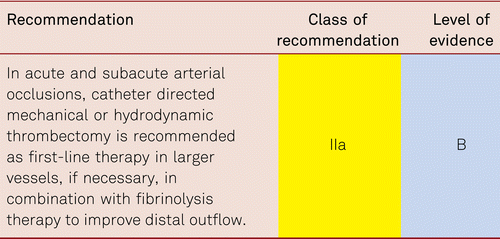
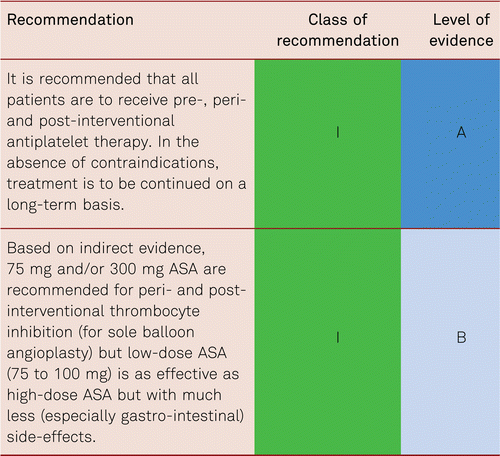
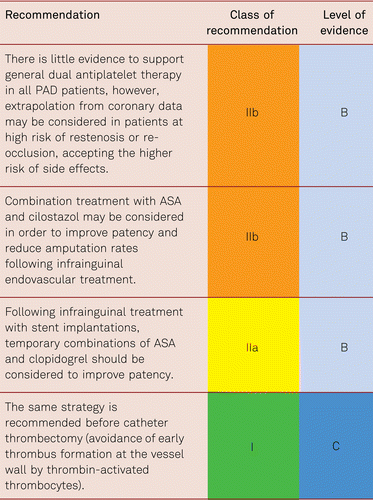
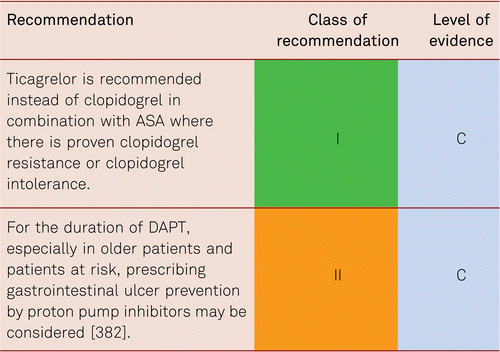
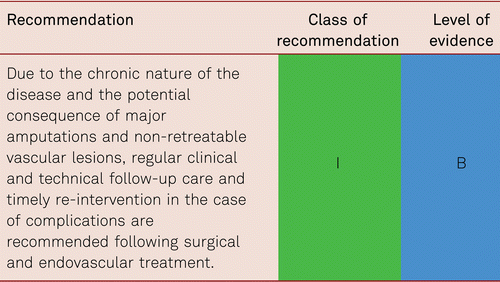
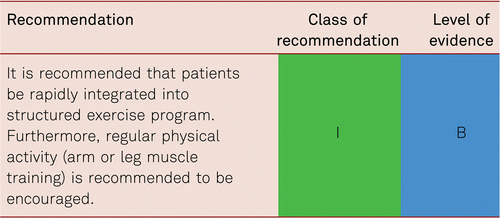
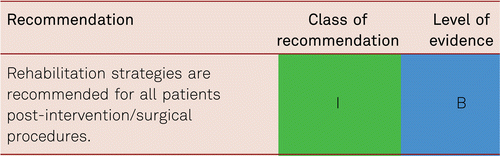
Interventional and/or surgical revascularization is possible in up to 70 to 90 % of patients with CLI [108–110]. This produces high healing rates and a significant decrease in rates of major amputation [48, 111]. At least in the medium term, interventional results prove comparable to the outcomes of vascular surgery when the TASC II criteria are observed [48, 112]. Table 4.1 summarizes the treatment recommendations in PAD.

5 Conservative treatment for PAD – Risk factor management
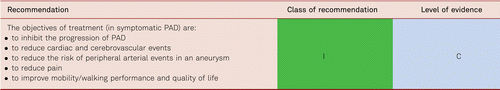
5.1 Objectives of conservative PAD treatment
The objectives of PAD treatment are to reduce the risk of future cardiovascular events (all Fontaine Stages) to improve walking performance, mobility and quality of life in Fontaine Stage II, and limb preservation, pain reduction and improved/maintain quality-of-life in Fontaine Stages III and IV.
This chapter deals with cardiovascular risk and its management in patients with PAD.
5.2 Cardiovascular risk management in PAD
Basic conservative treatment consists in monitoring of and treatment for cardiovascular risk factors for atherothrombosis. This covers regular physical activity, weight reduction in overweight patients, nicotine abstention in smokers, antiplatelet medication, as well as treatment for arterial hypertension, dyslipidemia and diabetes mellitus [113–117].
5.2.1 Smoking
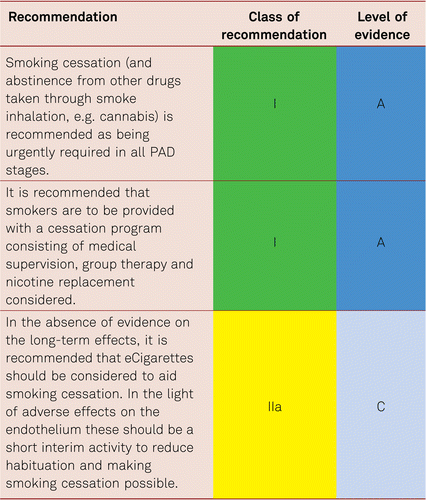
Smoking is at present the most important risk factors for PAD. The corresponding amount of exposure (number of pack years) is associated with the severity of PAD, a higher amputation rate, peripheral prosthetic bypass occlusion and mortality [118, 119]. The rate of amputations is markedly increased among smokers. Smoking cessation has been evidenced to impact on progression of PAD [120, 121], yet its significance for walking performance in intermittent claudication is less definite.
The rate of abstinence can demonstrably be improved with nicotine replacement preparations, formal cessation programs and bupropion [122–124]. Regularly and medically addressing the problem, along with intensive supervision, is the key to withdrawal. Other options, including group therapy or nicotine replacement preparations, should also be considered and can be combined with one another.
5.2.2 Depression
The presence of concomitant reactive depression in patients with PAD has become increasingly important [125]. The development of depression appears to limit quality of life and walking performance to a significant extent. In turn, reduced walking performance may possibly have a causative effect on the development of reactive depressive conditions. Data from interventional studies of antidepressants or psychiatric treatment for patients with PAD have not yet become available.
5.2.3 Dyslipidemia
Elevated total cholesterol concentrations, increased levels of low-density lipoprotein (LDL) cholesterol, triglyceride and lipoprotein (a), and decreased high-density lipoprotein (HDL) levels [126] are independent risk factors for the development of PAD. An inverse correlation has been shown between the level of LDL cholesterol and the ABI in patients with newly diagnosed PAD [127].
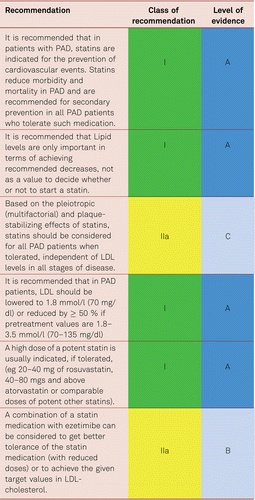
In the Heart Protection Study (HPS), simvastatin significantly lowered the rates of vascular and cardiac events in patients with PAD, irrespective of the presence of CAD at the beginning of the study [128–130]. This also applied to patients with so called ‘normal’ cholesterol values – indeed benefit was seen down to total cholesterol levels of 3.5 mmol/l. A cholesterol threshold value under which no benefit was detectable was not evidenced.
In prevention of vascular events and overall mortality, the most recent Cochrane analyses endorsed the benefits, cost efficiency and improved quality of life associated with statins, without accepting relevant undesirable effects even in low-risk patients [131].
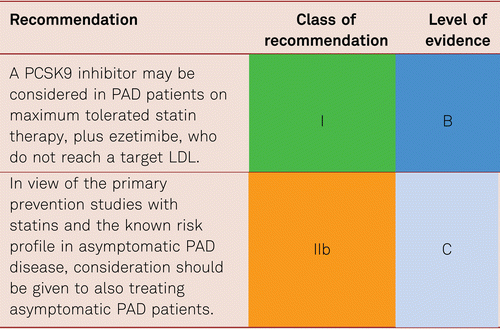
The extent to which LDL values should be lowered in PAD patients remains unclear, no prospective interventional studies have so far been carried out in PAD alone patients. However, the results of the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) supported the “LDL hypothesis” and suggested monitoring and the reduction of LDL values in patients at a high risk of cardiovascular disorders. Further the Fourier study [132] which enrolled 27,564 patients of which 13.2 % (3,642) had symptomatic PAD, all of whom were on statin therapy evaluated benefit in the preplanned PAD subgroup. The full study which was placebo controlled, of evolocumab, showed a significant decrease in the combined CV endpoints of MI, Stroke, and death (HR 0.80, 95 % CI 0.73–0.88) combined with a very good safety profile for very low levels of LDL. In the PAD subgroup, evolocumab significantly reduced the primary end point consistently in patients with PAD (hazard ratio [HR] 0.79; 95 % confidence interval [CI], 0.66–0.94, and also reduced the risk of major adverse limb events in all patients (HR, 0.58; 95 % CI, 0.38–0.88) [133]. This suggests that current targets are set to fall as these new drugs reach endpoint study conclusion.
The benefits of lipid lowering with statins and other lipid reducing agents in PAD is without doubt. Intriguingly, there is also some evidence in support of lipid lowering improving claudication. In the Scandinavian Simvastatin Survival Study (4S), simvastatin served to reduce the development of intermittent claudication in patients with coronary and stroke disease [128–134]. Further studies applying various doses of atorvastatin or simvastatin to treat patients with claudication yielded significantly improved pain-free or absolute walking distances compared to placebo after 3, 6, and 12 months. However, these studies examined small numbers of patient only [135–137].
Intensive statin therapy has been shown in retrospective studies to not only reduce CV mortality but also amputation [138, 139] and to enhance graft patency [140]. New data, recently available, shows that intensive statin therapy versus low intensity statin therapy better reduces adverse limb outcomes such as amputation [141].
Further consideration on additional therapy such as ezetimibe [142] and evolocumab [132], may need to be considered in this group which has such a high risk of further CV events.
The current European Society of Cardiology (ESC) guidelines recommend a lowering of LDL cholesterol depending on the overall cardiovascular risk to < 100 mg/dl (< 2.58 mmol/l) in high-risk patients and to < 70 mg/dl (< 1.81 mmol/l) in those at a very high risk including PAD patients (see the ESC Systematic Coronary Risk Evaluation [SCORE] system) [113]. To achieve these lower levels, monitoring of patient results, to ensure targets are reached, is crucial. Thus, it is not sufficient to merely start lipid lowering therapy, but the clinician must also ensure arrangements are in place for follow-up (e.g., in Primary Care) to ensure “treating to target”.
Identical target values can be recommended for women and men. However the current US guidelines have disassociated themselves from LDL titration as a target value [143]. PAD patients are considered very high-risk patients and are to be given “intensive” statin treatment with the aim to effectively and tolerably lower vascular risk. Medical Care has to focus on concordance, as non-adherence has shown to be a considerable problem in everyday practice.
The recent publication of the Fourier Study using the PCSK9 Inhibitor evolocumab, and its predefined sub-study of PAD [133], shows a significant decrease in both MACE and major adverse limb events (MALE). MACE in patients with PAD (hazard ratio [HR] 0.79; 95 % confidence interval [CI], 0.66–0.94 and without PAD (HR 0.86; 95 % CI, 0.80–0.93) and MALE (HR, 0.58; 95 % CI, 0.38–0.88; P = 0.0093).
61 % of PAD patients recruited had had an intervention, 40 % had diabetes, and the evidence showed the lower the LDL the better in terms of CV outcome with no lower limit being recommended and no overt side effects from this lower LDL. However, at present cost precludes us treating all patients, although a cost benefit analyses shows acceptable cost-effective thresholds [144] and so we recommend the following therapeutic strategy:
No data are available to evidence reduced morbidity and mortality by treating elevated triglyceride levels and low HDL cholesterol values.
Nicotinic acid reduces femoral atherothrombosis and delays coronary atherothrombosis in patients with PAD [145], yet has no additional verified benefits compared to statins in terms of reduced clinical endpoints and so cannot be recommended [146]. Although, omega-3 fatty acids has shown no effect on platelet activation or inflammatory parameters in patients with PAD, positive data are available for other risk populations [147–149]. However, there are no positive endpoint studies for patients with PAD [150, 151].
5.2.4 Diabetes mellitus
Apart from smoking, diabetic metabolic disorders are the most important risk factors for PAD progression. Every HbA1c increase in the magnitude of 1 % is associated with a 28 % increase in the relative risk for manifest PAD [152]. Diabetes elevates the risk of PAD by a factor of 3 to 4 and the risk of claudication by a factor of 2. A subgroup analysis of United Kingdom Prospective Diabetes Study (UKPDS) [40] in 3,884 patients showed a lower amputation rate with lower levels of HbA1c and/or lower levels of chronic hyperglycemia [152]. Another study showed significantly reduced walking performance and lower-limb mobility in patients with diabetes with PAD compared with patients without diabetes with PAD [153].
Among type 2 patients with diabetes under intensified treatment (diabetes stabilization, CSE inhibitor i.e. statin and antiaggregant administration), the Steno-2 study observed a 25 % relative risk reduction in amputation rates and a 10 % decrease in vascular interventions over 7 years [154]. Both type 1 and type 2 patients with diabetes benefit from improved glucose stabilisation in terms of reduced PAD progression. Current guidelines issued by the American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), and the European Society of Cardiology recommend the formulation of individualised treatment objectives for HbA1c together with the affected patients, considering the factors of age, comorbidity, hypoglycemic risk and patient’s preference. The aim is an HbA1c range of 6.5 % to 7.5 % to prevent subsequent complications [115, 155, 156]. In elderly vascular patients, a range of 7 % to 8 %, is seen as tolerable, while avoiding hypoglycemic events.
While 10-year follow-up data of the UKPDS has suggested a significant reduction in overall mortality, other studies have revealed no significant reduction in cardiovascular mortality by approximating the HbA1c value to normal by way of intensified diabetes treatment. These include Action to Control Cardiovascular Risk in Diabetes (ACCORD [157]), Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE [158]), Veterans Affairs Diabetes Trial (VADT [159]), Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation Post-trial Observational Study (ADVANCE-ON [160]).
The inconsistent database regarding the advantages of close-to-the-norm HbA1c stabilization is the reason for querying the lowest possible HbA1c target values and turning toward more individualised treatment strategies. Care for patients with vascular disorders and diabetic metabolic disorders requires interdisciplinary cooperation between vascular physicians and diabetologists.
Intentionally including pre-diabetes, the recent ESC/EASD guidelines classified patients with diabetes in groups at high and at very high risk for cardiovascular events. Individual specification of treatment objectives, measured according to HbA1c values and the avoidance of hypoglycemia, is the most important aspect of anti-hyperglycemic treatment. In individual cases, lifestyle interventions and treatment for comorbidities are to be applied.
5.2.4.1 Antidiabetic therapy in PAD
When it comes to diabetic therapy, the glucocentric view is increasingly taking a back seat. Rather, the treatment of cardiovascular comorbidities is of increasing importance. However, this is where there are considerable differences between the individual antidiabetic substances.
Biguanides
Metformin is the first-line oral antidiabetic drug in diabetics with PAOD, although the data is thin in this respect. A recently published study again demonstrates the positive effect on CV survival, but not on extremity retention and patency after peripheral revasculaization [161].
Sulfonylureas and Glinides
For both groups of substances no robust data on PAOD is available. Because of the relatively high risk of hypoglycemia and potential unfavorable effects in patients with coronary heart disease, these substances are hardly relevant [162].
DPP4 inhibitors
The cardiovascular endpoint studies SAVO-TIMI 53, EXAMINE, TECOS and CAROLINE show a non-inferiority of tested DPP4 inhibitors to the tested outcomes CV death, non-fatal myocardial infarction or stroke compared to placebo or glimepiride. In the SAVOR-Timi 53 study, however, significantly more hospitalization for saxagliptin was observed because of heart failure, so this substance should be used with caution in patients with known heart failure. Cardiovascular superiority or benefits in the presence of concomitant PAD are not proven [163–167].
GLP1 agonists
Both liraglutide, dulaglutide and semaglutide have been shown to have a positive effect on cardiovascular events such as fatal and non-fatal myocardial infarction and non-fatal stroke versus placebo in endpoint studies [168–170]. In the LEADER study Liraglutide reduces the amputation rate.
For semaglutide, however, there is an increased rate of microvascular ocular complications in combination with insulin, which is why this GLP-1 agonist should not be used in patients with diabetic retinopathy [169].
Thiazolidinediones (PPAR y agonists)
For Pioglitazone the PROACTIVE and the IRIS study show positive endpoint in cardiovascular survival in type 2 diabetes and prediabetic patients [171–175] In the PROACTIVE trial, amputation was also considered the primary endpoint. Here, however, no significant advantage over the control group could be observed.
SGLT-2 inhibitors
For the substances empaglifozin, dapaglifozin and canaglifozin, the EMPAREG outcome study, the DECLARE-TIMI study and the CANVAS study provide data for positively influencing cardiovascular outcomes such as CV death, fatal and non-fatal myocardial infarction and stroke [176–179]. The EMPAREG outcome study furthermore revealed a significantly reduction in amputations in PAOD whereas for canaglifozin a significantly increased amputation rate (mainly toe amputations) was observed. Although these are retrospective subgroup analyses, the use of canaglifozin in patients with diabetes and PAOD is currently not recommended.
Insulin
For basal insulin therapy, no endpoint studies are available for patients with PAOD. Insulin should be used in type 2 diabetics just in case of present cardiovascular complications, if possible, only after an optimized oral or GLP-1 based therapy.
In summery only empaglifozin and liraglutid have been shown to reduce amputations. Therefore, these substances should be considered in addition to metformin in patients with diabetes with previously known PAD [180, 181]. Screening for diabetes in patients with PAD should be carried out due to the close association between diabetes and PAD.
5.2.5 Hypertension
Hypertension is associated with an increased prevalence of PAD [182]. The increase in risk is modest but the high prevalence of hypertension with increasing age makes the contribution of elevated blood pressure to incident PAD in the general population important. In men 40–79 years old the hazard ratio for incident PAD in the presence of hypertension was reported to be 2.42 [183], whilst a 20 mm Hg increase in systolic blood pressure was associated with a 62 % increased risk for PAD in a large population study [184].
Antihypertensive therapy unequivocally reduces cardiovascular events and mortality. Current evidence suggests a further benefit of reducing systolic blood pressure to lower values than previously recommended [185–187]. Thus, current guidelines recommend a target blood pressure, if tolerated, of 120–129/70–80 mm Hg in patients below 65 years, and 130–139/70–80 mm Hg for older patients (with no upper age limit), also in people with concomitant cardiovascular disease (including PAD) and diabetes [188, 189]. A systolic blood pressure below 120 mm Hg should not be targeted as it may increase the risk for acute coronary events and the risk of harm outweighs the benefits, in particular in patients with advanced CAD and in the old.
Angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARB), calcium antagonists, and diuretics are all suited for blood pressure lowering treatment in patients with PAD[188, 189]. ACE inhibitors and ARB have been shown to reduce cardiovascular events in patients with arterial peripheral vascular diseases [190–192]. ACE inhibitors were proposed to improve symptoms in patients with intermittent claudication [193] but this view has been revised [194] while the calcium antagonist verapamil was shown to prolong walking distance in patients with PAD [195]. Studies in hypertension show somewhat less reduction in cardiovascular events with beta blockers than with other antihypertensive drug classes. Of note, beta blockers are well tolerated in PAD patients and are not contraindicated [196–198]. Thus, beta blockers are useful in PAD patients with concomitant cardiovascular disorders, where they are indicated.
Most patients require two or more drug classes to achieve target blood pressure. Thus, a blocker of the renin-angiotensin aldosterone system in combination with a calcium antagonist or a diuretic is recommended, adding the third drug, if needed, to achieve target blood pressure. If blood pressure remains uncontrolled adding spironolactone may be considered, which provides a greater reduction in blood pressure than adding an alpha blocker or a beta blocker [Williams 2015]. However, beta blockers are recommended when there are specific clinical indications.
5.2.6 Platelet aggregation inhibitors in symptomatic PAD
The clinical significance of decreased platelet aggregation in the secondary prevention of atherothrombotic vascular disorders is undisputed. However, large convincing studies showing a benefit of aspirin (ASA) in the reduction of vascular lesions in PAD have not yet been carried out. This applies to both PAD and the inhibition of progression in peripheral atherothrombosis. A primary meta-analysis carried out by the Antithrombotic Trialists’ Collaboration failed to detect a significant decrease in cardiovascular events in PAD patients who were taking ASA and who presented no further vascular lesions in other organs. So, while there are conclusive study results regarding the secondary prevention of cardiac and cerebrovascular events in patients with PAD with antiplatelet agents other than aspirin, the data base regarding the primary prevention of peripheral arterial events is insufficient and in part contradictory [199–201].
In a subsequent meta-analysis including many platelet aggregation inhibitors (ASA, clopidogrel, ticlopidine, dipyridamole, picotamide), a 23 % reduction in the relative risk for ischemic events was seen in all patients with PAD [202] but most patients received ticlopidine in these studies.
The benefit of life-long antiplatelet treatment in patients with PAD to prevent CAD or cerebrovascular lesions does seem convincing [9, 16, 203]. Historically ticlopidine has been investigated in several studies in PAD patients and has shown to reduce the risk of MI, stroke and vascular death [204]. However, its benefits are limited by very frequent gastrointestinal side effects and potential side effects as neutropenia and thrombocytopenia. Therefore, clopidogrel, another thienopyridine derivative, has replaced ticlopidine. Clopidogrel was investigated in the CAPRIE study in which it proved efficacy in lowering the rates of MI, stroke and cardiovascular mortality. The overall benefit in the PAD subgroup compared with ASA was a 24 % relative risk reduction [203]. In terms of numbers needed to treat (NNT) and compared to ASA, this corresponds to a total of 87 patients treated with clopidogrel over three years to avoid one additional cardiovascular event. In the current ESC PAD guidelines, clopidogrel was therefore recommended over aspirin in PAD patients [12] (Table 5.2.6.
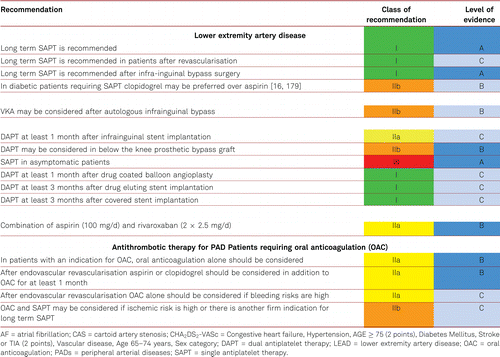
In the EUCLID study [205], ticagrelor and clopigogrel were equivalent in effect, though there were more drug discontinuations for side effects with Ticagrelol. Disappointingly, there was no ASA arm. However, the significance of the novel P2Y12 adenosine receptor antagonists (prasugrel and ticagrelor) for PAD is still a matter of ongoing research. The daily dose of clopidogrel in secondary prophylaxis is 75 mg.
Based on the TRA 2P – TIMI 50 study, vorapaxar, a thrombin receptor (protease-activated receptor, PAR-1) antagonist, was granted approval in the US for the secondary preventive treatment of CAD and PAD (in combination with ASA or clopidogrel, to the exclusion of patients with cerebral arterial occlusive disease or a history of intracerebral bleeding due to the increased risk of cerebral bleeding in this patient group). Approval in Europe is limited to CAD. TRA 2P – TIMI 50 study yielded significant benefits in terms of vascular complications and mortality for patients with PAD and CAD treated with vorapaxar, a protease-activated receptor 1 (PAR 1) antagonist, yet no gain for those presenting with cerebral arterial occlusive disease [206].
The combination of ASA and clopidogrel in high-risk patients with multiple risk factors and atherothrombotic manifestations (including PAD) – as well as in patients presenting with risk factors for cardiovascular disorders resulted in an increased bleeding risk and no benefit [207].
Combination treatment across the board has no statistically significant risk reductions in terms of MI, stroke or cardiovascular death. Therefore, combination treatment cannot be generally recommended for all patients with PAD. However, data from PEGASUS-TIMI 54 study [208, 209] prove that, in PAD patients with previous myocardial infarction, a selected high ischemic-risk subgroup, dual-antiplatelet therapy with aspirin and low-dose ticagrelor (60 mg b.i.d) is associated with a 5.2 % AR reduction in MACE and a reduction of MALE, while major TIMI bleedings were in excess of only 0.12 %. Therefore, DAPT with ticagrelor 60 mg b.i.d and aspirin may be considered in PAD patients with prior MI up to 3 years following the cardiac event. Evidence for prolonged treatment is currently lacking [12].
Evidence for dipyridamole in PAD is absent and is thus not indicated.
In summary, every symptomatic PAD patient should be given long-term treatment with platelet aggregation inhibitors, if there are no contraindications, as evidenced by the meta-analysis of the Antithrombotic Trialists’ Collaboration and data from the Swedish Ticlopidine Multicentre Study (STIMS) and the Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) study [42, 199, 207].
5.2.7 Platelet aggregation inhibitors in asymptomatic PAD
In asymptomatic disease, there is no evidence for the use of aspirin. Patients with diabetes with asymptomatic PAD given 100 mg daily of ASA, have shown neither reduced rates of cardiovascular events (lethal and nonlethal MI, stroke, cardiovascular mortality) nor reduced rates of major amputations compared to placebo-treated patients [17, 200, 201]. Amongst other reasons, the authors attributed this result to the wide application of statins in this high-risk group [167]. A large-scale, double-blind randomized population study covering a total of 28,980 Scottish residents found no clinically conspicuous cardiovascular disorders among 3,500 individuals with a decreased ABI score of ≤ 0.95. After a median treatment and follow-up period of 8.2 years, administration of ASA 100 mg/day orally had yielded no difference in the rate of cardiovascular events compared to placebo [210]. The benefit of antiplatelet agents in reducing peripheral arterial events has only been evidenced for patients given invasive endovascular treatment [42, 204].
5.2.8 Anticoagulants
From a peripheral arterial perspective, there is no indication for full dose INR lowering oral anticoagulation with vitamin K antagonists in patients with PAD, provided that there is no acute embolic event. However, the effect of a low dose antithrombotic therapy with the new oral anticoagulants in combination with ASA has been investigated.
In the COMPASS Trial [211], evaluating patients with CAD, and CAD and PAD, rivaroxaban and aspirin alone and in combination were studied in patients with stable atherosclerotic vascular disease. Those assigned to rivaroxaban (2.5 mg twice daily) plus ASA 100 mg daily, had a 24 % better total survival and cardiovascular outcome (HR 0.76 (0.66–0.86)) but more major bleeding events than those assigned to aspirin alone (HR 1.70 (1.40–2.05)). Rivaroxaban (5 mg twice daily) alone did not result in better cardiovascular outcomes than aspirin alone and resulted in more major bleeding events. Clearly a decision weighing up risks of CV event vs bleeding is required. The net benefit was 22 % overall risk reduction of the stable CAD/PAD population.
Additional analysis of the PAD subgroup (stable PAD, CAD with asymptomatic PAD and stable carotid stenosis patients) in the Compass trial population [212, 213] revealed, besides the 28 % general survival benefit, an additional significant 46 % reduction in major adverse limb events including major amputation (HR 0.54 95 % CI 0.35–0.82) for ASA 100 mg/d combined with Rivaroxaban 2 × 2.5 mg /d compared to ASA 100 mg/d and placebo. The net benefit was 28 % risk reduction for the PAD subgroup of COMPASS compared to the 24 % MACE reduction in the CAPRIE subgroup [203].
Rivaroxaban was also shown to be effective in these patients when suffering from mild to moderate heart failure [214]. An additional clinical trial (Voyager PAD) [215] comparing ASA 100 mg/d with and without 2 × 2.5 mg rivaroxaban/d − including symptomatic PAD patients undergoing a surgical- or catheter-based intervention- is underway. Also, other new oral anticoagulants are being tested in a clinical phase 3 trial in combination with ASA in this patient population. Table 5.2.8 gives an overview of the benefits of consistent treatment for cardiovascular risk factors in patients with PAD.
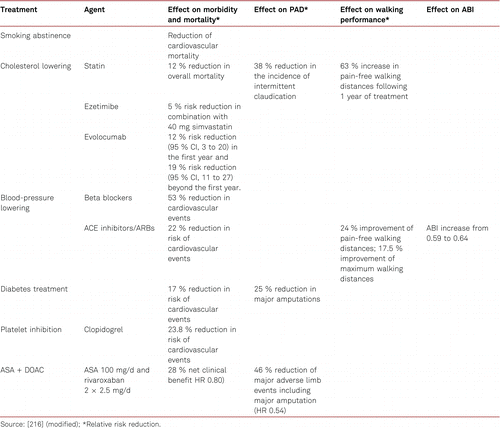
6 Conservative treatment for PAD: Symptom control
6.1 Conservative treatment for intermittent claudication
6.1.1 Walking training/rehabilitative sports in PAD
Walking training and physical activity are particularly important in the treatment of PAD. Structured walking is the most significant non-medical treatment for PAD along with treatment for cardiovascular risk factors. Cochrane reviews of the effectiveness of walking training, and additional benefits arising from supervised structured vascular training, have unambiguously shown the value of vascular sports [217–219]. Structured and supervised walking training has been shown to be superior to non-supervised walking. Walking training programs carried out for at least 3 months under supervision and/or according to instructions have shown both an increase in walking ability and a decrease in the severity of claudication [220, 221].
Structured walking training (e.g. in vascular sports groups) is effective when carried out at least three times a week for 30 to 60 minutes [222, 223]. Controlled studies in patients with claudication have demonstrated increases in walking distances amounting to approx. 200 % after 12 weeks [222–225]. Individual daily interval training for 60 minutes with 5- to 15-minute intervals was shown to be effective in prolonging walking distances. Intensities were chosen to allow claudication pain to develop [226–228]. In studies, functional long-term results of walking training also proved equivalent to vascular interventions alone [227, 228]. However, the effects of endovascular revascularization and walking training are additive [224, 229] and both treatment modalities may be part of an integrative approach. It has also been shown that upper limb training can benefit endothelial function in PAD patients. The favorable prognostic factors in walking training include the following criteria: less than 1 year following diagnosis of PAD, femoral artery occlusion, and good cardiopulmonary condition.
The same beneficial increase in pain free walking distance can be achieved in active smokers and nonsmokers. Thus, walking training is to be an inherent part of treatment for claudication.
A study [230] has demonstrated that endurance training with arm ergometers may produce improvements in pain-free and absolute walking distances that are comparable to the results of treadmill training, with similar calorie consumption and that such training thus could be an alternative or supplement to walking training.
Regular walking training and vascular sports lead both to improvements in walking distances and show additional beneficial modifications of glucose and lipid metabolism [231, 232].
Patients with pelvic artery stenoses and occlusions may also benefit from vascular training.
Taking into account all available study results, however, it should be noted that the number of patients evaluated in these studies is low [226].
As long as vascular lesions are not successfully recanalized by endovascular interventions or vascular surgery, walking training is not effective in stenoses of the deep femoral artery and occlusion of the ipsilateral superficial femoral artery. Thus, vascular recanalization should be performed in pelvic vascular lesions, femoral bifurcation lesions, and deep femoral artery stenoses or occlusions before basic treatment with walking training is subsequently introduced [224, 233].
In patients with high-grade stenosis or occlusion of the popliteal artery, exercise may have a limited effect on claudication because of the reduced options for collateralization. Therefore, revascularization may be recommended in this group prior to physical training, though remembering that surgical outcome may be associated with lower patency rates.
Alternative forms of training, e.g. of the upper limbs, should be attempted if walking training is not possible [220, 221].
Vascular sports groups or structured training programs have not yet been implemented everywhere. In this respect, there is considerable need for action when compared with the much greater numbers of coronary sports groups/exercise classes.
Approximately 50 % of PAD patients present with concomitant orthopedic and/or neurological disorders and/or cardiopulmonary functional deficits, which may impede walking training or render participation in structured vascular sports groups impossible. Such comorbidities are to be recorded prior to walking training and modifications introduced in order to facilitate participation in walking training for as many patients as possible.
6.1.2 Medical treatment
Controlled studies have shown an effective increase in walking distances after treatment with cilostazol and naftidrofuryl. Maintenance of walking distance improvements have been shown in 6- and up to 12-month treatments with cilostazol and naftidrofuryl, respectively. Treatment with these agents is to be discontinued if symptoms fail to improve after 3 months. A re-evaluation should then be carried out and, where appropriate, vascular training be continued throughout, as regardless of possible medical treatment with cilostazol or naftidrofuryl, patients with claudication should be encouraged to engage in physical exercises and regular walking training.
6.1.2.1 Cilostazol
Cilostazol, a type-3 phosphodiesterase inhibitor, is an oral substance. Apart from antiaggregant properties, effects of cilostazol on endothelial and smooth muscle tissue cells have been reported [234, 235].
Several large-scale, placebo-controlled studies have observed a significant increase in pain-free and maximum walking distances with this substance, in addition to superiority over pentoxifylline [236–238]. The most recent Cochrane analysis of data from 15 double-blind, placebo- or actively controlled studies, showed pain-free walking distances to improve by an average of 31 m in participants taking cilostazol 100 mg twice daily [239]. The fact that no reduction of cardiovascular events was shown for cilostazol was ascribed to low patient numbers, short observation periods and various forms of bias which together impaired the methodical quality of most of those investigations. Six RCTs have investigated cilostazol in patients with intermittent claudication. Administration of 100 mg twice daily was shown to yield an average increase in maximum walking distance of 76 % compared to 20 % with placebo. Along with statistically significant improvements in walking ability, quality-of-life improvements were also documented [240].
The main side effects, headache and diarrhea, are attributed to the vasodilating properties of cilostazol [241]. In a long-term observation in 1,885 patients, the Cilostazol: A Study in Long-term Effects, a large-scale safety investigation, showed no increase in cardiovascular mortality or bleeding tendency [242]. Cilostazol is contraindicated in patients with clinically manifest heart failure, unstable angina pectoris and MI, or coronary intervention within 6 months, as well as severe tachyarrhythmia [243].
Recent studies have demonstrated a marked reduction in restenosis rates following coronary and peripheral interventions with administration of cilostazol, in addition to standard therapy (dual antiplatelet therapy with ASA and clopidogrel) compared to placebo [244–250]. However, data available to the European Medicines Agency (EMA) indicate that the risk of bleeding may increase with concomitant administration of cilostazol and dual antiaggregation. Combinations with only one of the substances or sole application do not result in an increased bleeding risk [251]. Further as above, it is contraindicated in MI.
6.1.2.2 Naftidrofuryl
Six older studies with naftidrofuryl and a Cochrane analysis have summarized the benefits of long-term oral treatment [252]. The Cochrane Review concluded ‘Oral naftidrofuryl has a statistically significant and clinically meaningful, although moderate, effect of improving walking distance in the six months after initiation of therapy’. The Artériopathie Praxilene Indépendence Evénements Critiques (APIEC) study recruited 168 patients with claudication to be treated with naftidrofuryl or placebo for 1 year. The active group showed significant 107 % and 74 % improvements in pain-free and maximum walking distances, respectively [253]. Older studies had evidenced quality-of-life improvements for participants with claudication, which, however, more recent analyses failed to reproduce this [254]. A Cochrane analysis has suggested Cilostazol may be better than naftidrofuryl [239].
The effective recommended daily dose of naftidrofuryl is 200 mg tid.
Prescription of these substances can also be useful if, assessing the risk-benefit profile, arterial revascularization is too elaborate or risky or not accepted by the patient.
6.1.2.3 Others
There is no evidence that treatment with other vasoactive substances is effective in improving walking performance in claudication and are thus not indicated.
A systematic review comparing cilostazol, naftidrofuryl, and pentoxifylline in intermittent claudication analysed a total of 26 RCTs and identified naftidrofuryl as the most effective agent. Pentoxifylline was shown to be least effective in terms of improved maximum and pain-free walking distances. Maximum walking distances were increased by 60 % (95 % CI 20–114), 25 % (95 % CI 11–40), and 11 % (95 % CI 1–24) under naftidrofuryl, cilostazol and pentoxifylline, respectively, and pain-free walking distances by 49 %, 13 %, and 9 % respectively [255].
Statins may be useful in improving walking distance, but studies are small and need to be substantiated, as mentioned above [138–140].
Prostanoids, pentoxifylline, L-arginine, buflomedil and ginkgo biloba, have produced insufficient evidence for clinical application in claudication [238, 256–259]. The same applies to isovolemic hemodilution and other alternative treatments.
6.2 Conservative treatment for CLI
The primary objectives of treatment for CLI are alleviation of pain, healing of trophic lesions, avoidance of high-level amputations, improvement of limb functioning and quality of life, and medium-term survival improvement. These aims necessitate an interdisciplinary approach. The primary and most useful treatment option is revascularization.
Other approaches include analgesics administration, infection treatment, and the optimization of cardiac and pulmonary function. Monitoring of and treatment for cardiovascular risk factors is required in patients with CLI at least as much as any other patient with PAD [28, 67].
6.2.1 Treatment for ulceration
Multidisciplinary approaches are required to adhere to the major principles of treatment [260]:
- 1.Improvement of perfusion/revascularization as far as possible;
- 2.Local wound management: elimination of necrosis, moist wound milieu, treatment of infection;
- 3.Consistent and sufficient pressure relief, initially by immobilization, then with adequate expedients/relief shoes.
6.2.1.1 Infection
Local disinfection is preferred over local or systemic antibiotics [261]. Systemic antibiotic treatment is to be restricted unless there is the presence of systemic and or clinical signs of infection according to the PEDIS criteria, to avoid antibiotic resistance [262, 263]. In contrast, some patients with diabetes may show little inflammatory response and care should be taken not to deprive these patients of appropriate antibiotic therapy if there is a sudden clinical deterioration in their limb. Local antibiotics are not recommended. Patients with diabetic foot disease and osteomyelitis may be well treated with minor surgical procedures.
Non-revascularisable vascular lesions and unmanageable infection are the most significant predictors of major amputation. A prospective cohort study has shown increased rates of major amputations in spite of successful revascularization due to uncontrolled infections.
Normoglycemic metabolic status is to be achieved in patients with diabetes with CLI [7].
6.2.2 Prostanoids
Prostanoids are used when revascularizing procedures cannot be applied or have proven ineffective. Two randomized double-blind studies with prostaglandin E1 (PGE1) have demonstrated benefits in the reduction of ulcer size [264, 265]. A study with the stable prostacyclin analogue, iloprost, showed increased lower limb preservation and survival rates with prostanoid therapy [266]. As demonstrated in a meta-analysis, pain at rest and ulcer size may be reduced with 2 to 4 weeks of iloprost treatment. Moreover, after 6 months of observation, a 65 % rate of survival and leg preservation was seen with active medication compared to 45 % with placebo [267]. Another meta-analysis of placebo-controlled PGE1 studies showed that the compound significantly improved rates of ulcer healing and reduced pain compared to placebo [265]. Within 6 months, a significant difference in favor of PGE1 was also seen in terms of major amputations and mortality (PGE1: 22.6 %; placebo: 36.2 %) [265].
The most recent Cochrane analysis of prostanoid efficacy in patients with CLI participating in 20 older RCTs has identified positive effects with regards to ulcer healing (RR 1.54; 95 % CI 1.22–1.96), pain reduction (RR 1.32; 95 % CI 1.1–1.57) and improved amputation rates (RR 0.69; 95 % CI 0.52–0.93) compared to placebo or active control treatments. However, these studies failed to substantiate long-term benefits or decreases in cardiovascular mortality [268].
A recent RCT has failed to show any significant benefit from alprostadil in CLI [269].
Prostanoids are no longer listed as medical treatment options for patients with CLI in the guideline recommendations issued by the American College of Cardiology/American Heart Association (ACC/AHA) [67] and the National Institute for Health and Care Excellence (NICE) [68].
6.2.3 Stem Cell/Growth factor/Regenerative therapy
Cell and gene therapies are emerging as treatments for patients with CLI who are not eligible for endovascular or surgical revascularization. Several pre-clinical and early clinical studies suggested that different types of genes, angiogenic factors, and stem cells with regenerative potential and paracrine ability could improve blood circulation and tissue perfusion, and thus prevent amputation via the induction of capillary or collateral growth in a process called “therapeutic angiogenesis” [270, 271]. However in many cases larger studies have so far failed to confirm benefit.
6.2.3.1 Gene therapy
Different types of gene therapies (i.e. hepatocyte growth factor, fibroblast growth factor 1, and vascular endothelial growth factor) using different vector systems (i.e. non-viral, liposomal, viral) have been studied. They showed favorable safety profiles with low rates of adverse events.
The only large-scale phase III randomized trial with fibroblast growth factors (FGFs) applied in gene therapy for PAD proved to be negative [272]. The preliminary encouraging results of small case histories, case-control studies and a small, randomized controlled pilot study with genetically engineered non-viral FGF 1 (NV1FGF) in CLI have not been corroborated [271, 273, 274].
Similarly, investigation of gene therapy for other growth factors was discontinued over time due to missing proof of concept in various phase II trials.
6.2.3.2 Cell therapy
Although, many cell types have been tested, most clinical trials to date have relied on the use of adult autologous bone marrow-derived mononuclear cells or peripheral blood-derived mononuclear cells derived from the PAD patients treated [275, 276].
Despite several small early studies documenting the positive clinical outcomes of stem cell therapy, others have failed to confirm earlier results [277]. A recent meta-analysis of placebo-controlled trials using autologous bone marrow cells showed no advantage of stem cell therapy on the primary outcome measures of amputation, survival and amputation-free survival in patients with CLI [278]. Further, larger randomized placebo-controlled trials have so far failed to confirm earlier encouraging results [272]. The reasons may lie in the reduced availability and impaired quality of those cells deriving from patients with cardiovascular or vascular disease [279, 280].
Alternatively, mesenchymal stem cells have gained therapeutic interest in interventions aimed at tissue restoration because of their multipotent differentiation capacity and their cytoprotective and immunomodulatory effects [281, 282]. Also, they derive from young healthy individuals and patients do not have to undergo bone marrow aspiration. Large phase III trials with placenta-derived stem cells are currently ongoing in claudication and CLI patients.
6.2.4 Spinal cord stimulation
Available data on the efficacy of spinal cord stimulation (SCS) are inconsistent in terms of pain reduction, ulcer management and limb preservation in patients who are not eligible for revascularization and in non-responders to revasularisation [283, 284]. Among six controlled, yet non-blinded studies, the most recent Cochrane review identified an increased rate of limb preservation after 12 months (RR 0.71; 95 % CI 0.56–0.90) and pain reduction with SCS compared with conservative treatment. However, no significant effect was seen for ulcer healing. The complication risk under SCS amounted to 17 %, including implant difficulties in 9 %, stimulation difficulties necessitating re-implantation in 15 %, and local infections in 3 % [285]. Another meta-analysis covering five randomized studies failed to identify an advantage of SCS over conservative treatment alone [286].
6.2.5 Hyperbaric oxygen therapy
Hyperbaric oxygen therapy (HBOT) has been in use for several decades, especially for the treatment of diabetic wounds, including diabetic gangrene and diabetic foot ulcers as well as chronic or recurring wounds in which hypoxia has been demonstrated (as in critical lower limb ischemia). However, there is currently no clear evidence regarding its effectiveness.
In 2008 [287], a report issued by the Federaal Kenniscentrum voor de Gezondheidszorg (KCE, Belgian Health Care Knowledge Centre) attempted to describe the history of HBOT, the evidence for its use in different conditions, the economic value of its application, the costs for patients, hospitals and society. According to this report, even if HBOT was accepted by the European Committee for Hyperbaric Medicine (ECHM) and the Undersea & Hyperbaric Medical Society (UHMS) as a possible treatment for delayed wound healing, there was only low-quality evidence from small RCTs on the clinical efficacy of adjuvant HBOT in patients with diabetic ulcers in terms of major amputations in the medium term. Furthermore, there was very low-quality or no evidence for the efficacy of adjuvant HBOT for delayed wound healing other than that associated with diabetes.
According to a recent review and meta-analysis [288], there is low- to moderate-quality evidence suggesting a beneficial effect of HBOT when used as an adjunct to standard treatment for diabetic foot ulcers. HBOT is unlikely to be helpful in patients with severe uncorrectable ischemia because oxygen will not reach the ischemic area in a sufficient tension to stimulate angiogenesis.
In a recent, well designed randomized study [289], 107 patients with diabetes and foot lesions (Wagner grade 2–4) of at least 4 weeks’ duration who had comprehensive wound care were randomly assigned to receive 30 daily sessions of 90 min of HBOT (breathing oxygen at 244 kPa) or sham (breathing air at 125 kPa). At 12 weeks post-randomisation, 103 patients were available for endpoint adjudication. Criteria for major amputation were met in 13 of 54 patients in the sham group and 11 of 49 in the HBOT group (OR 0.91 [CI 0.37, 2.28]). Twelve patients (22 %) in the sham group and 10 (20 %) in the HBOT group were healed (OR 0.90 [0.35, 2.31]). HBOT in that study failed to offer an additional advantage to comprehensive wound care in reducing the indication for amputation or facilitating wound healing in patients with chronic diabetic foot ulcers.
6.2.6 Other medical treatments
Other medical treatments with vasoactive substances (buflomedil, pentoxifylline), heparin, or fibrinolytic drugs have shown no benefit in reducing amputation rates or promoting wound healing [290]. Evidence is still lacking for antiplatelet drugs in CLI have which failed to influence amputation-free survival, although they reduce the frequencies of cardiovascular events and mortality. However, the combination of an antiplatelet inhibitor with DOAC has proven to reduce limb amputations in a PAD population studied (low ABI, Claudication, previous intervention) by 70 % in the COMPASS trial [212].
In the presence of critical ischemia, interdisciplinary decisions regarding revascularization are to be made as quickly as possible [48].
Figure 6.1 summarizes the current guideline-adapted treatment recommendations for patients with CLI.
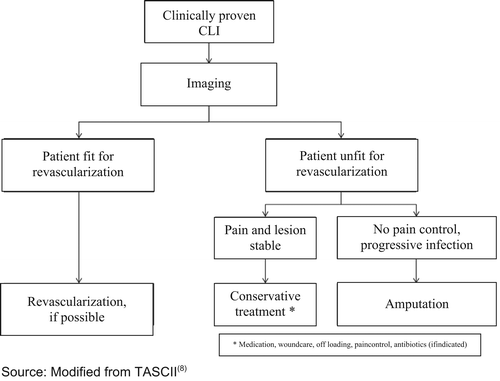
7 Interventional treatment for peripheral arterial disease
7.1 Introduction
Arterial reconstruction based on open surgical procedures or endovascular methods are symptomatic treatments that do not solve the underlying problem of progressive chronic atherosclerosis. However, they provide a reasonable and stage-adapted symptomatic treatment. The underlying atherosclerotic process still needs to be addressed.
The following are to be considered in the indications for interventional PAD treatment:
- •PAD stage according to Fontaine or Rutherford
- •Site, morphology and complexity of the vascular lesions
- •Concomitant disorders and procedural risk
- •Patient’s individual treatment wishes
The final aspect is particularly important, as several different treatments have all been shown to be feasible in the different stages of PAD. This is exemplified by the difficult choices to be made between conservative treatment and invasive endovascular or surgical treatment in the earlier stages of claudication. Also, in the treatment of critical limb ischemia (CLI), both endovascular and surgical measures can be used effectively, and it is crucial to discuss the advantages and disadvantages of each with the patient. Treatment recommendations must also consider the on-site availability of the various therapeutic methods, as well as the specific expertise of local interventionalists and surgeons.
Interventional treatment in PAD is characterised by rapid technological developments and high-level acceptance of non-invasive procedures among patients and operators. Therefore, clinical studies of interventions are urgently needed, e.g. in registries, and regularly assessed in terms of efficacy and safety. Defined questions can be addressed in the framework of multicenter studies.
7.2 Treatment according to clinical stages
7.2.1 Asymptomatic PAD
There is insufficient data as yet to confirm whether a prophylactic interventional procedure may positively impact the course of disease, limb salvage or survival in asymptomatic patients.
However, rarely interventional treatments can be considered in individual asymptomatic cases – this is most common in patients with diabetes with painless polyneuropathy and severe 3 vessel disease below the knee associated with hemodynamic critical limb ischemia at high risk for major tissue loss or amputation. Early re-intervention at an asymptomatic stage may also be considered in severe in-stent restenosis or bypass stenosis where total re-occlusion may be prevented by intervention (including graft failure) or distal thromboembolism. However, no data is available from evidence-based studies [38].
Table 7.1 stratifies potential patients at risk of foot lesions, thus guiding choice of management, even though subjective symptoms (claudication, pain at rest, necrosis) may not yet be present.

7.2.2 Intermittent claudication
In patients with intermittent claudication, regular and supervised exercise programs increase walking distances (so-called structured walking training) and may be equally efficient as revascularizations using angioplasty or vascular surgery [228]. However, structured exercise is less effective in pelvic and popliteal occlusions [224]. In many patients, pharmacotherapies are less effective compared to interventional therapies in the short term. Moreover, improving walking distances by exercise training contributes also to secondary cardiovascular prevention due to the cardiovascular benefits of increased exercise and endurance training. Thus, conservative treatment options should always be considered, especially following revascularization.
The Mild to Moderate Intermittent Claudication (MIMIC) trial showed improved long-term treatment success following initially successful interventional treatment in both iliac and femoropopliteal lesions when accompanied by subsequent structured vascular training [224]. Insufficient data is available regarding long-term results of crural interventions for claudication, as intermittent claudication is not generally an indication for crural interventions and walking exercise should be encouraged.
Following failure of conservative and/or medical treatment in patients with intermittent claudication, interventional treatment can produce short- and medium-term quality-of-life improvements along with improvement in walking ability [291]. Interventional or surgical measures may be considered in patients suffering from significant discomfort or pain, and when there is a need for longer walking distances because of the patient’s profession, or when explicitly desired by the given patient. A warning that a failed intervention may have serious limb consequences must, however, be given. Revascularizing measures can be considered, when improvement of mobility is still desirable but structured walking training is no longer feasible due to various circumstances, or no longer effective.
Impaired quality of life and individual disability due to decreased walking capacity are key to guiding recommendations for interventional treatments in patients with claudication.
However, interventional treatments below the knee should be considered in each individual carefully – this is most common in patients with diabetes with painless polyneuropathy and severe 3 vessel disease below the knee associated with hemodynamic critical limb ischemia at high risk for major tissue loss or amputation. These patients may also have foot claudication, but they are at risk of ulceration because of the neuropathy also. Structured exercise and symptomatic medical treatments are often insufficient in those with aortic or pelvic arterial lesions, and probably also those with popliteal occlusions, and are not recommended.
In this setting, and after excluding patient-related contraindications, endovascular treatment is the method of choice in the presence of appropriate vascular location and morphology (TASC A to C). Vascular surgery is primarily to be considered in femoral bifurcation lesions.
Revascularization is likely to improve walking capacity and therefore may reduce cardiovascular risk.
7.2.3 Critical limb ischemia
7.2.3.1 Basic aspects of treatment
The term CLI is used for patients with chronic ischemic pain at rest, and/or ulcerations and/or gangrene attributable to objectively proven arterial occlusive disease (see haemodynamic definition of PAD).
The short-term clinical results of surgical and interventional treatments are equivalent in clinical Fontaine stages III and IV [48]. Surgical treatments are associated with a higher morbidity and mortality, as well as higher costs, particularly in the presence of co-morbidities and increased perioperative risks for patients. Endovascular treatments are characterised by lower levels of invasiveness and complication rates, yet they more frequently require further treatment, mostly re-interventions due to restenosis or re-occlusion. On the basis of these advantages and disadvantages, individual risks and benefits should be assessed, and endovascular treatment given preference as the initial step.
The primary treatment objective in patients with critical ischemia is amputation-free survival, thus a decrease in both amputation and subsequent mortality rates among these patients at high cardiovascular risk is the aim. The long-term treatment results in patients with critical ischemia and those with intermittent claudication are thus different (see Table 7.2).

Reconstruction of unimpeded infrainguinal perfusion into at least one of the crural arteries is crucial to eliminate critical ischemia. This is a key precondition for the preservation or reconstruction of functional and pain-free limbs. Apart from revascularization, other factors have a substantial influence on healing and avoidance of amputations, including diabetes mellitus, end-stage renal failure, and a stage VI progressive tissue defect according to Rutherford.
Most patients with CLI present with a hugely impaired physical condition and multiple additional health problems, which makes the treatment of such patients a multidisciplinary challenge. Rapid management is a prerequisite to save legs and lives. The broad range of possible pathologies, the frequent frailty of the patient population with CLI, the frequent rapid progression, the urgent need for therapy, as well as the complexity of the different therapeutic options together result in the recommendation to refer those critically ill patients to specialised institutions.
In these specialized institutions, CLI patients should be evaluated using ankle pressure, always combined with toe pressure, measurements due to the high prevalence of medial sclerosis in CLI, followed by vascular ultrasound, as the majority of pathologies can be diagnosed with these methods without necessarily applying radiation or contrast media. Additional angiographic CT examination is recommended only in doubtful cases. Diagnostic angiography as the initial therapeutic tool is not advised because of the invasive nature of the procedure and the limited amount of information obtained with this technique (only luminal information, no vessel diameter, no additional wall information).
In addition to ultrasound examination, which reveals the cause of CLI, the degree of tissue impairment should also be evaluated (and documented) with noninvasive methods, such as pulse-volume recordings, toe oscillometry and toe pressure measurement, in addition to functional tests, including TcPO2 measurement or laser Doppler flowmetry (or intra-cutaneous capillaroscopy).
Prior to any interventional approach, interventionists are required to evaluate the patients’ general clinical state and review all diagnostic data. Particular attention is to be paid to the medication given on admission and during the hospital stay, basic laboratory findings (blood count, renal function, potassium, creatinine kinase, coagulation, thyroid-stimulating hormone level, blood glucose HbA1c), ultrasound or angiographic CT results (including access site and access path evaluation), possible additional medication before intervention (additional thrombocyte inhibition by dual antiplatelet therapy – possible pre-interventional loading dose), and the degree of required pain control.
Patients in uncontrolled pain (or presenting with impaired co-operation for other reasons) are to be evaluated for appropriate sedation prior to the intervention. A horizontal position of the patient during endovascular procedures worsens the perfusion situation at least initially and therefore, increases the pain level which can result uncontrolled agitation of the patient which may impede the planned interventional treatment or even make it impossible.
Provided with all possible information, interventionists then develop the interventional strategies that have the best chance of yielding good functional results with the lowest risks of acute or subsequent vascular or other (e.g., renal, pulmonary, cardiac) complications. The different techniques (ballooning and stenting including drug-eluting, catheter aspiration, thrombolysis, catheter directed mechanical and hydrodynamic thrombectomy, atherectomy, and others) may be used in each individual case depending on the given CLI situation. Many new interventional tools still require evidence of additional benefit in comparison with drug-eluting ballooning, catheter aspiration or thrombolysis. Even if benefits are not proven yet, many of these new tools have shown at least low risk profiles (no additional bleeding risk in comparison to thrombolysis, reduced peripheral embolization risk).
Because of the heterogeneity of CLI patients and the urgent requirement for treatment of the disease, it will be a considerable challenge to gain strong levels of evidence in this topic. Nevertheless, it should be the clear aim of further research. International prospective ESVM-initiated registries will be a first step.
7.2.3.2 Peripheral arterial disease – diabetic foot
Due to diabetic neuropathy, often the clinical stage usually does not correspond as well with hemodynamic and angiographic findings, being mostly much more severe than expected. In patients with diabetes, there are no limitations (with the exception of general contrast risks) to endovascular treatment for critical ischemia. Reconstruction of unimpeded tibial inflow is particularly important in cases of diabetic foot with a relevant ischemic component [292, 293]. Due to the characteristics of diabetic angiopathy with a focus on below-the-knee arterial changes, interventionists should be particularly experienced in treating long-segment diffuse lower-limb changes.
More than 40 % of CLI patients with diabetes suffer from renal insufficiency [272]. This is prognostically important and requires additional measures. Following extensive non-invasive diagnosis, selective angiography with PTA with the least possible amount of contrast medium and/or CO2 angiography should be performed, alongside sufficient prior hydration with consideration given to cardiac function, which may also be impaired due to concomitant coronary artery disease.
Co-morbidities and re-interventions are to be expected, due to the complex vascular disease with obstructions encountered at various levels with distal and multilevel lesions present particularly in patients with diabetes with end stage renal failure. Apart from several retrospective case series, only one randomized controlled study has indicated that if a tibial artery can be recanalized supplying the foot healing process of foot ulcers is significantly shortened following successful recanalization [294].
Recanalization with unimpeded tibial inflow into one or, if possible, both of the tibial vessels remains the primary objective [295]. Inflow alone does not seem to be sufficient – the target is a consistently patent vessel combined with wound therapy. The state of the vessels and clinical criteria should be considered in everyday triage processes.
7.3 Revascularization of chronic limb ischemia according to vascular beds
7.3.1 Aorto-iliac level
The Introduction of several large self-expanding stents and balloon-expanding covered stents and self-expanding aortoiliac stent grafts allow nowadays for the endovascular treatment of isolated aortic lesions or aortoiliac lesions. Recently, a new covered stent with very large diameters became available to treat isolated infra- or suprarenal aortic stenoses, which are typically highly calcified and thus requiring stents with high radial force. Combined with smaller covered stents or using such smaller covered stents as parallel grafts a complete reconstruction of the aortic bifurcation became possible. Using covered stents in the aortoiliac region allows for the revascularization with improved safety in the presence of thrombus and more complex situations like the combination of stenotic combined with aneurysmal disease. Revascularization strategies with multiple covered stents have been designed specifically for the reconstruction of the aortic bifurcation (CERAB) [296]. Yet, high thrombus burden in Leriche syndrome with increased risk of renal artery thrombosis or embolism still remains a surgical domain.
Case-control studies of interventional treatments for intermittent claudication have reported primary 5-year iliac patency rates of 60 % to 86 % on average (generally with stent implantation). Secondary patency rates were seen to be 80 % to 98 % [108, 110].
Technical progress in the methods of endovascular treatment has resulted in improved primary and secondary patency rates even in complex TASC C and D lesions. The primary long-term patency rates (5 to 7 years) following endovascular treatment for complex aortoiliac lesions (TASC II – C and D) are lower than those following bypass surgeries, yet the secondary patency rates after 4 and 5 years are comparable. This was shown by a systematic review of 19 cohort studies involving 1,711 patients [110].
The heterogeneity of the investigated population and the endovascular techniques used in these trials should be considered. The pooled technical success rate of 86 % to 100 %, clinical improvement was reported in 83 % to 100 %, and a mortality rate of 1.2 % to 6.7 % was reported in seven studies. The primary 4- to 5-year patency rates varied between 60 % and 80 % and thus fell below those given for bypass surgery. The secondary patency rates ranged between 80 % and 98 % and were thus comparable to the results of open surgery.
Furthermore, available data show higher primary patency rates following primary stent angioplasty than after primary balloon angioplasty with secondary stent implantation in such lesions. This is one of the results of a meta-analysis of 16 retrospective studies in a total of 948 patients with TASC C and D lesions. The pooled technical success rate was 92.8 % (93.7 % for C lesions and 90.1 % for D lesions) and the pooled primary 1-year patency rate was 88.7 % (89.6 % for C lesions and 87.3 % for D lesions). In terms of primary (vs. secondary) stenting for TASC C and D lesions, the technical success rate was 94.2 % (88 %) and the 1-year patency rate was 92.1 % (82.9 %). Long-term patency rates were thus significantly improved in the primary stent group as compared with selective stenting [108].
Recent data continue to support the high rates of technical success as well as the independence of patency rates from degrees of complexity (TASC II A to D). Published in 2013, a prospective non-randomized study conducted in Belgium and Italy reported 12-month patency rates following endovascular treatment for TASC A to D lesions of 94.0 %, 96.5 %. 91.3 % and 90.2 %, respectively. The rates were not statistically significantly different from one another and were in favor of primary as against selective stent implantation [297].
Endovascular treatment for aortoiliac lesions does not seem to lead to impaired outcomes for subsequent open surgery. In a retrospective cohort study (with a low number of participants (22 patients)), open surgery following unsuccessful endovascular treatment for aortoiliac lesions yielded no poorer results for up to 3 years compared to primary open surgery in 21 patients. Seven amputations proved necessary in the course of follow-up, all of which were in the primary surgical group [298].
7.3.2 Femoro-popliteal level
7.3.2.1 Endovascular vs. surgical care
The Bypass versus Angioplasty in Severe Ischemia of the Leg (BASIL) study is the largest randomized study to compare primary endovascular (n = 224) and primary surgical procedures (n = 228) with a long-term follow-up of up to 5 years in patients with CLI. Overall, neither method was seen to be superior in terms of mortality or amputation-free survival in the intention-to-treat population. Among patients surviving 2 years after randomization, those in the bypass group showed a survival rate improved by 7.3 months [299].
The only large-scale comparative investigation, the BASIL study, has however, several shortcomings. Instead of CLI, the investigators used the term “severe limb ischemia (SLI)”, an entity combining Rutherford IV to VI. No distinction was made between interventions or surgery above or below the knee. Therefore, this study is not helpful in making any definite statements regarding the treatment of a particular vascular region. Finally, only 10 % of all patients presenting with “SLI” at the participating centers were enrolled, indicating enrollment bias. Initial study results were published in 2005 and the percutaneous approach merely involved simple balloon angioplasty. In contrast, large clinical trials have now demonstrated the benefit of novel approaches, in particular for the femoropopliteal region. This was well recognized by the BASIL investigators who recently embarked on the BASIL II study investigating the clinical significance and cost-effectiveness of a ‘vein bypass first’ and a ‘best endovascular treatment first’ revascularization strategy for severe limb ischemia due to infrapopliteal disease [300].
With the progress recently made in the percutaneous treatment of femoropopliteal lesions, in particular with the advent of drug-eluting stents and balloons, the advantage of the percutaneous approach compared to the surgical approach may be expected to increase further.
A smaller retrospective cohort study in 139 extremities comparing primary PTA/stent with femoropopliteal polytetrafluoroethylene (PTFE) bypasses in long-segment femoropopliteal occlusions (TASC II C and D) yielded similar results with a slightly improved patency rate following endovascular treatment for TASC C lesions. Thus, endovascular intervention should be given preference over treatment with synthetic bypass surgery in the presence of increased surgical risk [301, 302]. This applies especially to patients beyond the age of 80 for whom interventional procedures are preferred over surgical methods due to increased comorbidity [303].
In the course of up to 4 years, virtually equivalent primary and secondary patency (SP) rates have been achieved using PTFE/nitinol stent grafts in percutaneous revascularization as compared to synthetic implants for open bypass surgery. A prospective randomized study yielded patency rates of 59 % (SP 74 %) following percutaneous revascularization and 58 % (SP 71 %) following application of synthetic bypasses [304].
7.3.2.2 Endovascular treatment with stents
For endovascular treatment in the femoropopliteal segment, in the absence of sufficient morphological and functional angiographic outcomes, the primary implantation of nitinol stents compared with primary balloon angioplasty and secondary stent implantation has resulted in the following:
- •No morphological or clinical advantage (in terms of primary patency or reintervention rates, respectively) in short lesions (< 5 cm) over the course of up to 1 year [305].
- •
- •
The initial recommendation, that for endovascular treatment for medium-length and long femoropopliteal lesions should be given preference to primary stent angioplasty with nitinol stents over balloon angioplasty with secondary stent implantations (bail out) is now not valid in the presence of newer revascularization methods outlined in the next chapter, in the presence of drug-eluting balloons.
Obstruction of the popliteal artery is frequently combined with occlusion of the superficial femoral artery and additional lesions of the trifurcation area. The popliteal segment constitutes an important potential connection segment for bypass anastomoses. This is the reason why there are few comparative studies with isolated lesions of the popliteal artery.
A prospective randomized investigation in 246 patients with de novo lesions of the popliteal artery has compared primary nitinol stent implantation and PTA with optional stent implantation in suboptimal results following PTA, which proved necessary in 25 % of treatments. One year after treatment, the primary patency rates following primary stenting and following PTA with bail-out stenting were identical, i.e. 67.4 % vs. 65.7 %, respectively [311].
7.3.2.3 Newer endovascular procedures
Restenosis remains the most serious problem associated with endovascular procedures in the femoropopliteal region. Many different approaches have been developed to reduce re-intervention. Stent implantation has reduced restenosis rates in comparison to balloon angioplasty alone. Still, primary patency rates, particularly after treating longer segments, have been seen to be only 60 % to 70 % after one year and only 30 % to 60 % after two years [312, 313]. This has led to the development of drug-eluting self-expanding stents which improve primary patency rates to 74.8 % after two years [314]. Five-year patency rates in the femoropopliteal vascular bed have been reported in the Zilver PTX Randomized Trial for the use of drug-eluting stents (DES). Approximately 91 % of patients had claudication; 9 % had critical limb ischemia. The 5-year primary end points of event-free survival and patency showed superiority of primary DES in comparison with PTA sustained through 5 years. Clinical benefit (freedom from persistent or worsening symptoms of ischemia; 79.8 % versus 59.3 %, P < 0.01), patency (66.4 % versus 43.4 %, P < 0.01), and freedom from reintervention (target lesion revascularization, 83.1 % versus 67.6 %, P < 0.01) for the overall DES group were superior to standard care in non-randomized comparisons. Similarly, clinical benefit (81.8 % versus 63.8 %, P = 0.02), patency (72.4 % versus 53.0 %, P = 0.03), and freedom from target lesion revascularization (84.9 % versus 71.6 %, P = 0.06) with provisional DES were improved over provisional BMS. These results represent > 40 % relative risk reduction for restenosis and target lesion revascularization through 5 years for the overall DES in comparison with standard care and for provisional DES in comparison with provisional BMS [315].
However, stents, in particular long self-expanding stents, impose constant mechanical strain on the vasculature. This applies even more to oversized stents.
A significant improvement in the results of endovascular procedures has been achieved with the introduction of drug-eluting balloons. Currently, 2-year results are available from two large randomized controlled trials and from a single-arm pivotal trial using various drug-eluting balloons.
At 24 months, patients treated with drug-eluting balloons (DEB) (frequently also named drug coated balloons), showed in the Randomized Trial of IN.PACT Admiral Drug Coated Balloon vs Standard PTA for the Treatment of SFA and Proximal Popliteal Arterial Disease (INPACT SFA) significantly higher primary patency when compared with non-coated PTA (78.9 % vs. 50.1 %; p < 0.001). The rates of CD-TLR were 9.1 % and 28.3 % (p < 0.001) for the DEB and PTA groups, respectively. Both groups showed similar functional improvement at 2 years, although DEB patients achieved this level of function with 58 % fewer re-interventions [316].
In the single-blind, randomized LEVANT-2 trial, 476 patients with symptomatic intermittent claudication or ischemic pain while at rest and angiographically significant atherosclerotic lesions were randomized to angioplasty with a paclitaxel-coated balloon or to standard angioplasty. At 12 months, the rate of primary patency among patients who had undergone angioplasty with the drug-eluting balloon was superior to that among patients who had undergone conventional angioplasty (65.2 % vs. 52.6 %, P = 0.02). The proportion of patients free from primary safety events was 83.9 % with the drug-eluting balloon and 79.0 % with standard angioplasty (P=0.005 for noninferiority). There were no significant between-group differences in functional outcomes or in the rates of death, amputation, thrombosis, or reintervention [317].
Taken together, current evidence and expert opinions primarily support the use of drug-eluting balloons after vigorous pre-dilatation, as the results are at least as good if not better than with stenting. An advantage is that nothing is left behind in arteries that may cause constant strain on the vasculature. Focal stenting should be limited to areas of relevant dissection. The concept of focal stenting, however, is currently derived from indirect evidence only and is the subject of ongoing clinical studies. Therefore, it can only be considered on the basis of expert opinion. Self-expanding stenting to primarily treat highly calcified arterial obstructions does not appear to be appropriate. In the Surgical vs. Percutaneous Bypass (Super-B) trial, with a wire-interwoven stent that possesses the strongest radial force of all available stents, inadequate vessel preparation led to increasing rates of restenosis. In this trial 325 patients (264 intention-to-treat and 64 roll-in subjects) were enrolled. Treated lesion lengths were 7.8 cm ± 4.3 cm in the trial with chronic total occlusions comprising 24.6 % (65/264) of subjects. Freedom from clinically driven target lesion revascularization (CD-TLR) at 12 months was 89 %, at 24 and 36 months it was 84 % and 82 % respectively. At 2 and 3 years, freedom from CD-TLR in minimal compression was 86.7 % and was 90.0 % for moderate compression. At 2 and 3 years, freedom from CD-TLR for moderate elongation was 81.8 % and 78.2 %, and for severe elongation was 63.4 % and 42.3 %, respectively. Fractures were distinctly uncommon with this stent with a single fracture event in the 36-month follow-up period. Thus, the interwoven nitinol design stent is a stent that achieves an excellent primary patency but further maintains the durability of the stent through 36 months. Optimal stent deployment remains critical to the performance of this stent device and requires optimal vessel preparation [318].
Drug-eluting balloon angioplasty is superior over plain balloon angioplasty and stenting is superior over plain balloon angioplasty. According to current evidence, plain balloon angioplasty without using drug-eluting balloons and/or without using stents cannot be justified for endovascular treatment of the femoropopliteal segment any longer.
A number of tools are available to achieve this goal even in heavily calcified vessels, including different atherectomy systems and scoring balloons. However, large controlled clinical trials using these systems in this indication are still missing. The recommendations of particular endovascular techniques to achieve an optimal result are thus to be considered as a focus for research in a currently very active field.
Also, new stent devices are being currently tested minimizing stent material left behind while preserving arterial flexibility by using multiple 6 mm Tack stent rings focally. Tacks were used in 130 patients with post-PTA dissections (74.0 % ≥ grade C) following standard balloon angioplasty. The 12-month patency was 76.4 %, and freedom from target lesion revascularization was 89.5 % [319].
A current trial evaluates Tack mini stents in combination with DEB.
The meta-analysis of Konstantinos et al. (2018) [320]. raised concerns about the safety of paclitaxel coating because of an excess mortality in the paclitaxel treated patient population. The investigation of the authorities, especially the FDA are on-going and for now the recommendations of the FDA (https://www.fda.gov/medical-devices/letters-health-care-providers/august-7-2019-update-treatment-peripheral-arterial-disease-paclitaxel-coated-balloons-and-paclitaxel), medical associations and national authorities is to restrict the use of these devices as far as possible and to use alternate techniques. Also, patients should be informed about ongoing investigations ahead of use and have the choice to object the use of such paclitaxel-eluting devices.
In the meantime, independent adjudication found zero deaths attributable to one of the frequently used 0.035″ paclitaxel-coated balloons [321]. An analysis with German health claims data of 9.2 million insurants was evaluated for long-term mortality after use of paclitaxel-based drug-eluting devices in lower limb arteries from their introduction to the market in Germany in 2007 until present. In this cohort, 64,771 patients were included who underwent 107,112 endovascular procedures in which 200,681 devices, thereof 23,137 paclitaxel-based DES and DCBs, were applied. A time-dependent multivariable Cox regression analysis was conducted accounting for each single device that was applied during the entire study period. This allowed to assess mortality hazards which might change over time and to assess also for various combinations of all devices and multiple interventions [322]. In summary, taking any performed intervention and all applied devices into account, there was no signal of increased mortality over 11 years of follow-up after use of paclitaxel-based DCBs or DES in lower limb artery disease [323].
7.3.3 Infra-popliteal level
Randomized comparative data regarding interventional and surgical revascularization techniques are limited to the results of the BASIL study which, however, included few infrapopliteal recanalizations [112]. This prospective randomized trial showed no difference in amputation-free survival up to 5 years after primary endovascular vs. primary open surgery. Endovascular procedures were associated with increased rates of re-intervention and open surgery with increased rates of morbidity and mortality, in addition to higher costs.
Comparable patency and leg-salvage rates following endovascular vs. surgical revascularization have been computed from retrospective cohort analyses [323]. The study cohort consisted of 1,023 patients with critical ischemia, of whom 262 and 761 received endovascular treatment and open surgery, respectively. Following risk adjustment, the 5-year rates of limb salvage were 75.3 % after endovascular treatment and 76.0 % after bypass procedures. Amputation-free survival amounted to 37.7 % and 37.3 %, respectively. Systematic analyses of the literature reveal that both endovascular treatment and open surgery yield an approximately 80 % leg-salvage rate 3 years post-therapy [324]. Improvements in catheter techniques have contributed to a high success rate in recanalizations, even in long infrapopliteal occlusions (> 10 cm) in the stage of critical ischemia [104, 325, 326].
Case series have rarely investigated angioplasty alone in infrapopliteal arteries (below the knee arteries – BTK) in the presence of intermittent claudication. For example, in the DEBELLUM trial 122 lesions were treated: 92 (75.4 %) SFA, 30 (24.6 %) BTK. Twenty (40 %) patients presented multilevel concomitant femoropopliteal and infra-popliteal lesions. Late lumen loss (LLL) was 0.64 ± 0.9 mm in the Drug Eluting Balloon (DEB) group vs. 1.81 ± 0.1 mm in the control group (P = 0.01). In non-stented segment LLL was 0.63±0.9 mm (DEB) vs. 1.70 ± 0.6 mm (PTA), P < 0.01. In the stent subgroup was LLL 0.65 ± 0.2 mm (DEB) vs. 1.91 ± 0.3 mm (PTA), P < 0.01. BTK the overall LLL was 0.66 ± 0.9 mm (DEB) vs. 1.69 ± 0.5 mm (PTA) (P = 0.03). The overall TLR was 12.2 % for DEB and 35.3 % for PTA (P < 0.05). Amputation rate was 4 % (DEB) vs. 12 % (PTA), P = 0.36. Fontaine stage increased (from II b to I) 80 % DEB vs. 56 % PTA (P < 0.05). The Debellum trial used In.Pact DEB balloons, which can be considered efficient to reduce restenosis rate [327].
Another investigation has reported a relatively high rate of major complications (5.8 %) and a poor long-term result with a re-occlusion rate of 33.7 % after one year [328]. Thus, exclusive use of angioplasty cannot be consistently recommended, although still considered in individual cases, after carefully assessing risks and benefits and providing comprehensive information.
Several controlled randomized studies have addressed the issue as to whether balloon angioplasty or stent angioplasty are primarily indicated in the presence of infrapopliteal vessels and have failed to evidence superiority for primary stenting [329, 330]. A meta-analysis of 16 non-randomized clinical trials has demonstrated no advantage of primary Bare metal stent (BMS) implantation over PTA alone, yet showed a tendency toward improved results with Drug eluting stent (DES) implantation versus BMS and PTA alone [331] but this is only true for focal lesions in segments suitable for stent implantation.
7.3.3.1 Drug-eluting stents (DES)
With endovascular recanalizations of less extensive (< 3 cm) infrapopliteal lesions, the short-term application of DES (≤ 1 year) has resulted in reduced rates of both restenoses and re-interventions compared to those achieved with non-DES. Individual studies have indicated a medium-term decrease in amputation rates after one year [331–337]. A comparative investigation analysing the revascularization of infrapoplietal de novo stenoses in 161 patients using sirolimus-eluting stents (SES) showed a tendency towards a 2.6 % lower amputation rate after approximately 3 years as against 12.2 % with the use of BMS [336]. Two meta-analyses of randomized studies in 2013 comparing DES and BMS have yielded similar results in 1-year outcomes [334, 335].
The use of sirolimus-eluting stents (SES) in secondary stent implantation following insufficient balloon angioplasty has resulted in decreased restenosis and re-intervention rates compared to the use of non-DES. However, no effect has so far been evidenced as to the rate of amputations [338]. A systematic review of 18 non-randomized trials has established both higher primary patency rates and lower restenosis rates following SES implantation after failed balloon angioplasty compared to non-DES or paclitaxel-eluting stent (PES) implantation [339]. Again the influence of DES on the clinical endpoints of limb salvage and amputation-free survival has not yet been sufficiently explored. In addition, their application is always limited because of their relatively short length and the crushing risk in regions of increased motion and bending.
In a newer meta-analysis of randomized trials, the 1 to 48-month follow-up outcomes of different endovascular treatment strategies in below-the-knee (BTK) arterial segments in critical limb Ischemia (CLI) patients were evaluated. Twelve trials including 1,145 patients were identified, with 90 % of patients having CLI. Six BMS versus PTA and two DES versus PTA trials showed low-quality evidence of equal efficacy. One trial, comparing DEB with PTA, showed moderate-quality evidence of improved wound healing (RR 1.28; 95 % CI: 1.05 to 1.56), improvement in Rutherford classification (RR 1.32; 95 % CI: 1.08 to 1.60), and lower TLR-rates (RR 0.41; 95 % CI 0.23 to 0.74) and binary restenosis (RR 0.36; 95 % CI 0.24 to 0.54) in diabetic patients after 12 months. Amputation and death rate did not differ significantly. For DES versus BMS, most trials showed equal efficacy between strategies [340].
More recent comparative studies of bare-metal stents (BMS) and drug-eluting stents (DES) have shown primary 1-year patency rates in mixed patient groups with intermittent claudication and CLI to range between 48 % and 66 % with PTA and no stent implantation. In a meta-analysis of randomized trials, investigating the outcomes of percutaneous revascularization with primary drug-eluting stenting in patients with atherosclerotic disease of infrapopliteal arteries compared with plain balloon angioplasty or bare-metal stents (BMSs), a total of 611 patients from 5 trials were randomly assigned to DES (n = 294) versus control therapy (plain balloon angioplasty/BMS implantation, n = 307). At a median follow-up of 12 months (IQR: 12 to 36 months), DESs reduced the risk of target lesion revascularization (odds ratio [OR]: 0.31; 95 % confidence interval [CI]: 0.18 to 0.54; p < 0.001), restenosis (OR: 0.25; 95 % CI: 0.15 to 0.43; p < 0.001), and amputation (OR: 0.50; 95 % CI: 0.26 to 0.97); p = 0.04) without a significant difference in terms of death (OR: 0.81; 95 % CI: 0.45 to 1.49; p = 0.50) and Rutherford class improvement (OR: 1.36; 95 % CI: 0.91 to 2.04; p = 0.13) versus control therapy. Thus, in focal disease of infrapopliteal arteries, DES therapy reduces the risk of reintervention and amputation compared with plain balloon angioplasty or BMS implantation without any impact on mortality and Rutherford class at 1-year follow-up [335].
In a systematic review of the literature of all studies comparing stent treatments of infragenicular vessels in patients with chronic lower limb ischemia, the search identified 4 randomized clinical trials and 2 observational studies reporting on 544 patients (287 treated with DES and 257 treated with BMS). Primary patency, freedom from target lesion revascularization, and clinical improvement at 1 year were significantly higher in the DES recipients compared to patients treated with BMS (OR 4.511, 95 % CI 2.897 to 7.024, p < 0.001, NNT 3.5; OR 3.238, 95 % CI 2.019 to 5.192, p < 0.001, NNT 6.0; and OR 1.792, 95 % CI 1.039 to 3.090, p = 0.036, NNT 7.3, respectively). No significant differences in limb salvage and overall survival at 1 year were identified between the groups (OR 2.008, 95 % CI 0.722 to 5.585, p = 0.181; OR 1.262, 95 % CI 0.605 to 2.634, p = 0.535, respectively). Thus, this analysis demonstrated superior short-term results with DES compared with BMS, expressed by increased patency and freedom from target lesion revascularization [334].
Most recent data on DES implantation achieve over 80 % one-year patency. Percutaneous stent angioplasty was performed in 128 limbs in 114 patients presenting with 320 vascular lesions. Lesions with up to 6 cm in length and at least one patent vessel below the obstruction were treated; 341 sirolimus-eluting Cypher(R) stents (diameter of 2.5–3.5 mm; length of 18–33 mm) were implanted. Follow-up examinations were performed up to 18 months post-interventionally using clinical examination, ankle-brachial index calculation, and color-coded Duplex sonography. The 6, 12, and 18 months primary patency rate as controlled by Duplex sonography was 89.8, 84.2 and 83.3 %, respectively; 77.6 % of the lesions healed post-interventionally. The cumulative limb salvage rate was 95.6 % [341].
7.3.3.2 Bio-absorbable (metal) stents
For revascularization of infrapopliteal arteries, no morphological or clinical benefits have as yet been achieved with bioabsorbable metal stents compared to balloon angioplasty applied alone [342].
Also everolimus coated bioabsorbable scaffolds could not show an advantage compared to non-absorbable DES in the coronaries [343].
7.3.3.3 Paclitaxel-coated balloons
Two initial randomized pilot studies have indicated positive 6- and/or 12-month data regarding patency rates following treatment for infrapopliteal lesions with DEBs compared to uncoated balloons. One of the trials investigated patients with diabetes exclusively (see section 6.4.2.4) [327, 344]. A subsequent, larger-scale prospective controlled study comparing angioplasties in lower-limb arteries using paclitaxel-coated balloons vs. non-DEBs failed to confirm such advantages for DEBs [345]. With a median length of 10 to 13 cm, lesions were treated in 358 patients with critical ischemia. After one year, the rates of clinically induced re-interventions showed no difference between treatments with DEBs and non-DEBs (9.2 % vs. 13.1 %). There was a trend, however, showing the rate of major amputations following DEB was higher (8.8 % vs. 3.6 %).
Such DEBs were removed from the market instantly as a consequence of available study results. The technical problem apparently was not with the drug but rather with the balloon design and the production process. Data from one randomized trial and 2 several single arm studies conducted with other DEBs have in the meantime became available.
In the randomized BIOLUX P-II trial a novel paclitaxel-coated drug-eluting balloon (DEB) versus an uncoated balloon (percutaneous transluminal angioplasty [PTA]) in de novo or native restenotic lesions of the infrapopliteal arteries in patients with claudication and critical limb ischemia was compared. 72 patients were randomized 1:1 to either a Passeo-18 Lux DEB (n = 36) or Passeo-18 PTA balloon (n = 36). Follow-up assessments were scheduled at 1, 6, and 12 months, with angiographic assessment at 6 months. The primary safety endpoint (a composite of all-cause mortality, target extremity major amputation, target lesion thrombosis, and target vessel revascularization at 30 days) was 0 % in the DEB group versus 8.3 % in the PTA group (p = 0.239). The primary performance endpoint (patency loss at 6 months) was 17.1 % in the DEB group versus 26.1 % in the PTA group (p = 0.298), and major amputations of the target extremity occurred in 3.3 % versus 5.6 % of the patients at 12 months, respectively. Thus, the Passeo-18 Lux DEB has been proven to be safe and effective in infrapopliteal lesions with comparable outcomes to PTA [346].
In a retrospective review 208 patients suffering from either severe claudication (38.6 %) or critical limb ischemia (CLI; 61.4 %) in 220 limbs were analysed. Almost two-thirds (140, 63.6 %) of the 220 target lesions were total occlusions, and 37 (17.8 %) of all patients had occlusion of all 3 BTK vessels before intervention. Over a median 9-month follow-up, target lesion revascularization occurred in 15.9 % of patients with an average time to first reintervention of 8 months. In total, 39 amputations were performed in 31 limbs. However, 17 of these amputations were preplanned minor amputations below the ankle; only 9 (4.1 %) major amputations occurred corresponding to 6.6 % of the CLI cohort. Freedom from the composite of death or major amputation was estimated as 92 % and 85 % at 6 and 12 months, respectively, by Kaplan-Meier analysis. In the full cohort, improvement of at least 1 Rutherford category was seen in 130 (59.1 %) limbs after 1 year or at the last follow-up, with 104 (80.0 %) of those limbs showing an improvement of ≥ 2 categories. From this single-center experience, the Lutonix® DEB shows therapeutic promise in a disease state where new treatment options were needed [347].
In another retrospective analysis, 21 patients (15 men; mean age 66.6 ± 11.2 years) were followed-up for 356.5 ± 159.2 days and 90.47 % had 12-months follow up data available for analysis. TcPO2 increased (14.3 ± 11.6 mmHg to 53.8 ± 11.7 mmHg; p < 0.05). Limb salvage rate was 100 %, and 90.4 % of patients achieved the combined endpoint of reduction in ulcer size/depth or complete healing.
DEB had superior efficacy (MALE+post-operative death, amputation free survival, freedom from re-intervention, limb salvage and survival rates), while attaining superior or equivalent safety (Major Adverse Limb Events, major adverse cardiovascular events and Amputation) endpoints for the overall, modified clinical and anatomical high-risk groups. Thus, use of Lutonix® DEB was safe and effective for recurring infrapopliteal disease. It outperformed the objective performance goals (OPG) for CLI patients with clinical and anatomical high-risk features [348]. The significance of drug-eluting balloons in angioplasty for infrapopliteal arteries have not yet been sufficiently assessed. The results of the ongoing randomized trials in lower limb arteries are awaited.
7.3.3.4 Angiosome concept
Direct revascularization of the artery covering an ischemic region (angiosome) is the primary aim, as it tends to yield better results in terms of ulcer healing and leg salvage than indirect revascularization [294, 349–352]. Revascularization of the crural arteries that provide the ischemic lesions via collaterals, however, has also been seen to be effective in ulcer healing and leg preservation [353]. Direct revascularization also serves to promote the healing of ischemic ulcers which, however, is substantially and negatively influenced by the presence of terminal renal failure, diabetes or advanced tissue defects as in Rutherford stage VI [354].
In patients with diabetes, the recanalization of tibial arteries seems to be more efficient in allowing for healing of ischemic tissue lesions than the recanalization of the fibular (peroneal) artery [355]. Implementation of several crural artery revascularizations does not improve clinical long-term results [356].
7.3.4 General considerations
Interventional treatment in patients with critical ischemia has high rates of technical and primary clinical success up to 65 % to 95 % depending on the complexity of vascular lesions, length and degree of calcification in the affected vascular stenosis/occlusions [108, 110, 297, 299, 307, 331]. Following interventional therapy for infrapopliteal vascular occlusions in critical ischemia, the primary patency rate is 40 % to 60 % after 3 to 5 years, respectively. In turn, secondary patency rates following re-interventions are up to 80 %, with over 90 % leg-salvage rates in small case series [109, 292, 299, 325, 357].
Re-occlusions in treated vessel are not necessarily associated with ulcer recurrences, as the healing process in critical ischemia generally only requires a temporary improvement of cruropedal perfusion. The quicker and more completely cruropedal revascularization is successfully carried out, the lower the risk of major amputation.
Multilevel disease is frequent in CLI. Lesions of the aortoiliac vascular bed should be treated first in order to improve inflow into the limb. At the same time, or as a second step, treatment for critical ischemia also requires the elimination of obstruction frequently present in the femoropopliteal and/or cruropedal outflow tracts. In the presence of combined lesions in the inflow and outflow segments, these should be treated next in an attempt to reconstruct an unimpeded pedal inflow over at least one crural artery. Due to improved guidewires and catheters available and depending on the length of occlusion and degree of calcification, primary technical success rates are up to 80 % to 90 %, even in long lesions. In this situation, application of retrograde transpopliteal, transpedal and transcrural recanalization may prove useful in individual cases.
However, when treatment of proximal lesion may technically obstruct future endovascular treatment of distal lesions as in the event of stenting of common iliac stenosis in the presence of proximal femoral stenosis or occlusions, the strategy may be inverted.
In critical ischemia there will be also an ‘endovascular first’ therapy in regions, where the best treatment (surgical vs. endovascular) is still under debate. So, it can be reasonable to treat long iliac or common femoral stenoses or occlusions by endovascular techniques, to have the option to treat lesions below the pelvic or common femoral artery level.
There are now many new devices for the interventional therapy of vascular lesions causing CLI. The evidence is still not robust but in specialized institutions some of the devices are already an indispensable part of the interventional armamentarium, making therapies faster and safer than before [290, 358–361].
7.3.5 Emergency catheter therapies in acute limb ischemia
Four entities of atherosclerotic acute limb ischemia need to be distinguished:
- 1.acute worsening of pre-existing chronic limb ischemia
- 2.acute re-occlusions after earlier (stent) angioplasty or bypass surgery
- 3.acute occlusion in thromboembolic disease
- 4.acute occlusion in compression syndromes
The underlying cause of acute worsening of chronic PAD is very different from the other causes. In most cases, CLI presents as a multifocal disease with critically reduced tissue perfusion of limb or foot. Acutely or subacutely, aortoiliac or femoropopliteal atherosclerotic disease may lead to plaque rupture (with or without hemodynamically relevant pre-existing stenosis), resulting in an acute arterial occlusion or, more rarely, distal cholesterol embolisation as a cause of CLI worsening. Depending on underlying or concomitant diseases, the cause of threatened tissue perfusion may be located at different levels of the vasculature. Thus, initial therapy may vary within a broad spectrum of options.
In contrast, distal embolization due to atrial fibrillation can occur in the absence of PAD and is also very common in the aging patient, – confounding with worsening of pre-existing PAD may be therefore possible. Other underlying causes are arterial aneurysms also leading to distal embolism and/or acute vascular occlusions at the site of, mostly, popliteal aneurysms. Furthermore, compression syndromes with mostly bilateral manifestations may lead to acute vascular occlusions.
Finally, secondary to previous endovascular procedures (mostly with stent) or bypass surgeries re-occlusions may occur quite suddenly from embolization or even dissection.
In general, the more distally the perfusion problem is located, the more difficult is its treatment. Because of the broad range of possible pathologies and the emergency character of acute limb ischemia, RCT evidence for the different treatment options is still required but difficult to generate.
Depending on the underlying cause, therapy will be endovascular or surgical. Thrombosed and occluded popliteal aneurysms need surgical therapy to avoid further distal embolism by exclusion or resection of the aneurysm and circumvention using an extra-anatomic bypass or venous interposition. However, in cases where the embolising popliteal aneurysm is still patent, an endovascular exclusion with a flexible covered stent is possible and has the advantage of possible combination with selective thrombolysis of the distal emboli.
A total of 72 popliteal aneurysms (PAA) were treated in 70 patients. The majority of PAA were asymptomatic (78 %). Sixteen cases (22 %) had a symptomatic PAA, of which seven (44 %) presented with acute ischemia. Median follow-up was 13 months (range 0–63 months). Primary patency rate at 1 year was 83 % and after 3 years 69 %; primary assisted patency rate was 87 % at 1 year and 74 % after 3 years. Secondary patency rate was 88 % and 76 % at 1 and 3 years, respectively. There were no amputations during follow-up. Thus, endovascular treatment of PAA with heparin-bonded stent grafts is a safe treatment option with good early and mid-term patency rates comparable with open repair using the great saphenous vein [362]. However 90 % of patients in this study did not have acute symptoms and serious cardiopulmonary disease was an exclusion criterion. Aspiration thrombectomy is indicated if the limb is acutely ischemic.
Recently, multilayer flow diverting bare cobalt alloy self-expanding stents were used to exclude peripheral and visceral artery aneurysms. In a multicentre, prospective, voluntary registry, 54 patients underwent multilayer stent deployment for peripheral (n = 35) or visceral aneurysms (n = 19). Among the 54 lesions, 44 had a total of 53 side branches. There was no aneurysm rupture. Six (11.1 %) stents occluded over the 1-year period. Cumulative primary and secondary patency estimates were 86.9 % and 90.7 % at 1 year. The cumulative side branch patency was 96.1 % and the freedom from all complications was 83.0 % at 1 year. Complete aneurysm thrombosis was recorded in 42 (93.3 %) of 45 patients at 1 year. Percent diameter reduction was 15.5 %, 3.8 %, and 11.0 % at 1, 6, and 12 months (p < 0.05), respectively [363]. In acute ischemia aspiration thrombectomy may be preferred over thrombolysis because of safety concerns.
Arteries occluded by external compression will need surgical external decompression and venous interpositions.
Following distal thromboembolism in the absence of aneurysmal disease or compression, catheter directed mechanical or hydrodynamic thrombectomy [364, 365] and/or selective fibrinolysis are therapies of choice in the absence of contraindications. If there are severe motor or sensory impairments immediate surgical thrombectomy/embolectomy is still the first line therapy. Rotational atherectomy may be preferable in those patients at high risk of bleeding.
Catheter directed mechanical or hydrodynamic thrombectomy has the advantage of avoiding a bleeding risk associated with thrombolysis and therefore, in most cases does not need the use of an intensive care bed.
In the first publication, 98 patients (65 men; mean age 66 ± 9 years, range 47–90) with 100 thrombotic occlusions (mean age of occlusion 31 ± 33 days, range 0–140) measuring an average of 21 ± 11 cm long (range 2–40) were treated with rotational thrombectomy (Rotarex®). There were 33 acute (≤ 14 days) thrombotic/embolic native artery occlusions (group I), 58 subacute/chronic (> 14 days) native artery occlusions (group II), and 9 acute bypass graft occlusions (group III).
Slightly less than half the arteries (48 %) were stented. Primary success (residual stenosis < 30 %) was achieved in 92 % (94 % for group I, 93 % for group II, and 78 % for group III; 100 % for the ipsilateral approach, 56 % for the crossover approach). 8 vessel perforations and 7 cases of peripheral embolization allowed further therapies to be considered and readiness for selective thrombolysis and covered stents became a prerequisite to perform this procedure safely [365]. Catheter directed mechanical or hydrodynamic thrombectomy is thus the treatment of choice for acute and subacute/chronic thrombotic infra-aortic occlusions of native vessels and bypass grafts but has been used also in visceral arteries and subclavian and vertebral arteries.
In acute limb ischemia, compartment syndrome must be screened for, and fasciotomy carried out where necessary.
8 Care of the patient undergoing vascular interventions
8.1 Peripheral interventions: Peri- and Post-interventional medical follow-up
Endovascular treatment for PAD has undergone dynamic developments over the past years. It is now mostly first-line treatment in multi-morbid high-risk patients with Stage III and IV disease, and increasingly in complicated Stage II disorders. The main objective of peri- and post-interventional care – improved patency rates – can be achieved both by preventing early or later-stage post-interventional arterial thrombosis, and by avoiding restenosis. The following chapter thus focuses exclusively on anticoagulation and antithrombocytic/antiplatelet treatment used to avoid arterial thrombosis.
Local vascular inflammation following PTA or stent implantation is mainly responsible for medium- and long-term restenosis processes [366]. Early thrombosis and occlusion mostly develop as a result of dissection or local arterial platelet activation. Rates of restenosis or re-occlusions depend not only on vascular morphology and the kind of endovascular technique or implanted stent chosen, but also on the vascular region. For example, stent implantations into the femoropopliteal regions are associated with a markedly higher restenosis risk than into the iliac vessels. This is possibly caused by higher levels of local inflammation in the muscular arteries of the femoral vascular bed as opposed to less severe inflammatory reactions in the elastic arteries of the pelvic vascular bed [367].
Restenosis or re-occlusion resulting from balloon dilatation may develop in three successive phases of different etiology:
- •Elastic recoiling which mostly occurs within 24 hours after intervention and can be avoided with stent implantation.
- •Thrombus formation which occurs usually within the first weeks or months depending on the length of the lesion and the implanted material used.
- •Neointimal hyperplasia which is detectable around three to six months, by activation of smooth muscle cells and synthesis of extracellular matrix [368].
Disorder-related chronic inflammation of the vascular walls may also affect restenosis and the risk of occlusion following primarily successful interventions. Examples of this include advanced chronic renal failure and diabetic metabolic disorders, which are particularly important. The restenosis risk is significantly higher in patients with renal failure and/or diabetes compared to those without these comorbidities [369]. Evidence-based recommendations on the most beneficial antithrombotic treatments to be used in peripheral interventions are limited by the lack of corresponding randomized controlled clinical trials and are adapted from the coronary interventional trials.
8.1.1 Antiplatelet drugs
Platelet activation is elevated in patients with PAD, indicating an increased intra-arterial thrombotic tendency [370]. Antiplatelet treatment is thus recommended for all patients with symptomatic PAD, regardless of secondary prophylactic interventions, in order to reduce associated cardio- and cerebrovascular morbidity and mortality (see Chapter 5). However, this chapter focuses on the peri- and post-interventional period.
Two randomized trials investigated whether antiaggregant treatments may improve patency rates following peripheral PTA. The first trial randomized 199 patients following angioplasty of the femoropopliteal vasculature to one of three treatment arms: Dipyridamole (225 mg) combined with high-dose ASA (990 mg) vs. dipyridamole with low-dose ASA (300 mg) vs. placebo. Both arms on active treatment showed better patency rates compared with the placebo group, of which the high-dose ASA group showed statistically significant improvements [371]. Following balloon dilatation of the pelvic or femoral vasculature, the second clinical trial randomized 223 patients to combination therapy with ASA (50 mg) and dipyridamole (400 mg) vs. placebo. The primary patency rates were identical in both groups and no benefit of antiaggregant treatment was identified. However, the study was limited in the fact that the placebo arm included a larger share of PTAs of the iliac arteries which per se show a better outcome [199, 372].
One single study examined whether combination treatment with ASA and dipyridamole was superior to ASA alone [373]. After 14 days of treatment, no significant difference was seen between the groups in terms of primary patency rates following femoral or popliteal PTA.
Several older studies explored whether high-dose ASA is better than low-dose ASA in preventing re-occlusion [371, 374–377]. Six months after PTA, higher-dose ASA (900 to 1,000 mg) yielded no advantage, yet the number of gastrointestinal side effects increased with the dosages of ASA. ASA dosages between 50 mg and 300 mg are effective in follow-up care.
In clinical practice, as well as in most interventional trials, a temporary Dual Anti-Platelet Therapy (DAPT) with ASA and clopidogrel is recommended after using drug-eluting balloon angioplasty, implantation of bare metal stents or implantation of drug-eluting stents following femoropopliteal and/or infrainguinal treatment.
Combination treatments of ASA and thienopyridines (ticlopidine, clopidogrel) are frequently used in everyday clinical practice, following balloon angioplasty with stents or drug eluting techniques, especially when the femoropopliteal or tibial arterial vasculatures are affected. Only one single double-blind randomized trial (the MIRROR trial) with 80 patients compared monotherapy with ASA (100 mg/d) and dual antiplatelet treatment (ASA 100 mg/d + clopidogrel 75 mg/d) for 6 months, and subsequently for 1 year, following endovascular treatment with stent PTA in the femoropopliteal segment, concluding that the drug should be given longer. However, the primary endpoint was the local concentration of platelet-activating β-thromboglobulin and cluster of differentiation of 40 ligand (CD40L), as well as the rate of clopidogrel resistance. The clinical endpoints of re-intervention (target lesion revascularization) and bleeding were predefined merely as secondary endpoints. Re-interventions at the target lesions were significantly less frequent under dual antiplatelet treatment (p = 0.04). In terms of absolute frequency, however, re-interventions with only two vs. eight patients strongly limited the validity of the study. Bleeding complications under dual treatment were not increased [378].
ASA in combination with thienopyridines was shown to be superior to ASA alone or combined with oral anticoagulants (OAC) in terms of patency rates following the setting of coronary arterial stents [379–381]. The issue remains questionable as to whether these results can be extrapolated to peripheral interventions.
Likewise, the question whether a loading dose of clopidogrel (300 mg vs. 600 mg) should be given prior to planned peripheral interventions with stent implantations is unclear, as no trial has addressed this question. Finally, apart from the small study mentioned above, no randomized investigation has addressed the necessary duration of time to give dual antiplatelet treatment following endovascular treatment with stent implantations. No evidence-based conclusions can be drawn in this connection.
The Clopidogrel and Aspirin in the Management of Peripheral Endovascular Revascularization (CAMPER) trial was initiated in the USA to investigate the combination of ASA and clopidogrel vs. ASA alone following femoropopliteal angioplasty. However, the study was discontinued after one year due to insufficient randomization, possibly because an excessive number of patients in the USA had received that off-label combination treatment after femoropopliteal angioplasties. Thus, it is doubtful whether such a study could ever be carried out in the future [374]. Furthermore, in applying dual antiaggregation, it is crucial to consider that combination treatment in patients at a high vascular risk and with severe comorbidity is likely to be associated with an increased bleeding risk as compared to monotherapy [199]. However, data from PEGASUS-TIMI 54 study [208, 209] prove that, in PAD patients with previous myocardial infarction, a selected high ischemic-risk subgroup, dual-antiplatelet therapy with aspirin and low-dose ticagrelor (60 mg b.i.d) is associated with a 5.2 % AR reduction in MACE and a reduction of MALE, while major TIMI bleedings were in excess of only 0.12 %. Therefore, DAPT with ticagrelor 60 mg b.i.d and aspirin may be considered in PAD patients with prior MI [12]. In initiating dual antiplatelet treatment and deciding on the duration of combined therapy, due attention should be paid to the localization and kind of stent used, as well as the individual patients’ bleeding risks.
The trials addressing cilostazol treatment in patients with intermittent claudication and femoropopliteal disease following endovascular interventions are also small though prospective. These studies have compared re-intervention rates following administration of ASA alone or combined with cilostazol. One of these trials showed substantial methodical deficiencies, i.e. no blinding, unclear randomisation, and small patient numbers. Revascularization was carried out in 36 subjects, 16 under cilostazol and 20 in the control group. Re-interventions proved more frequently necessary in patients not given additional cilostazol (14 vs. 6; p = 0.041) [249]. Other studies on additional cilostazol following femoropopliteal interventions with and without stents went in the same direction. Five retrospective case series and two small prospective studies showed a reduced restenosis rate after treatment with cilostazol [382].
A recent, large-scale retrospective population-based analysis in the USA covering data from more than 23,000 subjects with PAD investigated cilostazol following endovascular and open surgical revascularizations [383]. ASA was given to 20,335 patients as a secondary prophylaxis and 1,999 received additional cilostazol. Compared with the patient group not given cilostazol, subjects receiving pre- and/or post treatment cilostazol showed significantly reduced rates of restenosis and major amputation.
On condition that there are no contraindications and in the presence of adequate tolerability, combination treatment with ASA and cilostazol can be considered to improve patency and reduce amputation rates following infrainguinal endovascular treatment. In individual cases, this especially applies to patients at a high risk for major amputation after vascular reconstructions. However, cilostazol is not approved for this indication in some EU countries.
From a pathophysiological perspective, and based on data pertaining to the coronary arteries, temporary dual antiaggregation with a combination of ASA 100 mg/d and clopidogrel 75 mg/d may be considered useful in the absence of evidence concerning the peripheral vasculature. The duration of dual treatment and the issue whether clopidogrel loading doses are effective or necessary in the periphery both remain unclear.
The use of more recent platelet aggregation inhibitors (especially ticagrelor in combination with ASA 100 mg/d) in this indication had a comparable effect to the combination of ASA and clopidogrel [205]. However, their use following cardiology interventions demonstrated benefits regarding efficacy leading to the recommendation of preferring ticagrelor over clopidogrel in patients with acute coronary syndromes [12]. In patients with clopidogrel resistance, ticagrelor and prasugrel may be used replacing clopidogrel.
Using batroxobin, a fibrinogen reducing serine protease, a small pilot study detected a significant reduction in the rates of restenosis and re-occlusion, with a consistent rate of amputation, among patients with diabetes following crural interventions [385]. Adding batroxobin to ASA 100 mg/d in this indication, a subsequent larger-scale randomized study in 129 subjects observed reduced restenosis and increased leg salvage rates together with significantly reduced major amputation rates in the group treated with combination ASA-batroxobin after 12 months [386].
8.1.2 Heparins
Unfractionated and low-molecular-weight heparins show both antithrombotic and antiproliferative effects [387]. The standard treatment with unfractionated heparins (UFHs) is usually started immediately before and during the course of intervention. The objective is to prolong the activated clotting time (ACT) to 200 to 250 seconds [7]. In a monocentric open study, Strecker et al. administered reviparin (3,500 IU 2x/d for 24 days) and ASA (100 mg/d) to 42 patients who had received femoropopliteal stent implants following PTA failure [388]. Compared with a historic control group treated with UFH, the 1- and 2-year rates of primary patency were higher with reviparin (88 % and 74 %) than with UFH (81 % and 50 %).
In a comparative study, 172 patients were treated with body-weight-adapted doses of nadroparin for 7 days or UFH i.v. after peripheral intervention of the femoropopliteal arteries. At 3 weeks, 3 months and 6 months post-intervention, the group given low-molecular-weight heparin showed a significantly lower number of re-occlusions than the group given UFH [389].
In another study, 275 subjects were treated with dalteparin (2,500 IU 1x/d for 3 months) and ASA (100 mg/d) or ASA alone [390]. After the 12-month study period, the restenosis and re-occlusion rates were identical in both groups (44 % in the ASA-dalteparin group vs. 50 % under ASA monotherapy). Only the subgroup of patients with critical limb ischemia (CLI) and long occlusions under additional treatment with low-molecular-weight heparin showed significantly higher patency rates after 12 months. The underlying cause is remaining thrombotic material which is often seen following endovascular revascularization of complex, long lesions, particularly long chronic occlusions. Remaining thrombus is also seen in most cases following selective thrombolysis and following rotational thrombectomy. Although not studied systematically, general experience demonstrates fewer early re-occlusions using intravenous heparins over several days post-intervention. No randomized studies have addressed this indication (and have probably lost their legitimacy due to long-term experience).
8.1.3 Oral anticoagulants (OAC)
Three randomized controlled studies investigated the extent to which OACs would constitute an alternative to antiaggregant treatment (ASA and dipyridamole) following peripheral angioplasty of the femoropopliteal and tibial vasculatures. A total of 438 patients were randomized. In all trials the groups treated with OAC (Vitamin K Antagonists) showed lower rates of arterial patency yet significantly higher bleeding rates (HR 1.79; 95 % CI 1.3–4.6) [199, 201, 391, 392]. Antiplatelet drugs should thus be first-line in interventional treatments, as long as there are no other imperative indications for OAC which are mostly deriving from cardiology co-morbities such as atrial fibrillation.
8.1.4 Direct oral anticoagulants (DOACs) in PAD
Provisional results presented from the pivotal randomized Edoxaban in Peripheral arterial disease (ePAD) trial [393] compared the effect of dual antiplatelet therapy (ASA and clopidogrel for 3 months after PTA, followed by ASA 100 mg/d for another 3 months) with a combination of ASA 100 mg/d and edoxaban 60 mg o.d. for 3 months after PTA followed by 3 additional months of ASA 100 mg/d. In all categories, the ASA/edoxaban combination tended to show lower bleeding rates and better outcome after 6 months (lower rate of major adverse cardiac events). This proof of concept is currently being evaluated in larger trials with edoxaban.
Additional analysis of the PAD subgroup (stable PAD, CAD with asymptomatic PAD and stable carotid stenosis patients) in the COMPASS trial population (X 46) revealed, besides the 28 % general survival benefit, an additional significant 48 % reduction in major adverse limb events including reduction in major amputation (HR 0.54 95 % CI 0.35–0.82) for ASA 100 mg/d combined with Rivaroxaban 2 × 2.5 mg /d compared to ASS 100 mg/d and placebo.
The current VOYAGER trial investigates rivaroxaban in combination with aspirin following PTA [215] (Table 8.1.4).
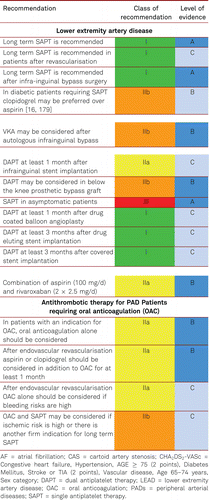
8.2 Surgical interventions: Peri- and post-operative medical follow-up
Many methods are available in terms of surgical revascularization, including thromboendarterectomy, ring stripping, thrombectomy, femoral bifurcation plasty, profundaplasty, and bypass procedures applying both venous and synthetic grafts. In particular, in the presence of multilevel disorders, complex hybrid procedures can be employed, involving a combination of balloon dilatation and, if required, stent implantation for proximal lesions and simultaneous bypasses for distal lesions [7].
The patency rates of all bypass procedures in the lower limbs require adjuvant platelet inhibition treatment, regardless of the type of bypass used [199]. Early occlusions following venous and synthetic bypasses are mostly caused by technical problems associated with blood flow disorders. Medium-term and late occlusions may be caused by neointimal hyperplasia in the graft itself or anastomosis. Alternatively, arteriosclerosis may progress in the native vascular bed [394]. The high thrombogenicity of the inner surface of synthetic bypasses makes the principle difference between the thrombotic occlusions of venous and synthetic bypasses. While venous bypasses are coated with endothelium and thus less thrombogenic, synthetic bypasses rarely show completely developed endothelium layers [199].
The ongoing Voyager PAD trial [215] will show, whether there will be in the surgical group an advantage of postoperative dual treatment of low dose DOAC (rivaroxaban 2 × 2.5 mg/d) and ASA 100 mg/d over ASA 100 mg/d alone.
8.2.1 Antiplatelet agents following surgery
Whether antiplatelet treatment is to be initiated preoperatively is a matter of ongoing controversy. In two out of three studies, preoperative onset showed improved postoperative rates of patency [395, 396]. Although, the third study failed to substantiate improved bypass patency following postoperative initiation [397], the evidence is slightly in favor of a preoperative onset of treatment with antiplatelet agents [199].
Antiplatelet treatment is recommended to improve patency rates following lower-leg bypass surgery, whereas it is more effective in synthetic grafts than in autologous conduits [7, 398–400]. A meta-analysis covering 11 randomized clinical studies carried out before 1990 showed administration of antiplatelet agents to significantly lower the risk of bypass occlusion by 32 % [42]. Published in 1999, a meta-analysis of five trials explored ASA (alone or in combination with other antiplatelet drugs) vs. placebo in patients with infrainguinal bypasses. Bypass occlusions were seen in 28.4 % of 423 subjects given antiplatelet treatment and in 36.6 % of 393 subjects given placebo. The relative risk of infrainguinal graft occlusion was significantly lowered (RR: 0.78) in patients under ASA [401].
In patients already receiving dual antiplatelet treatment, the intra- and perioperative risks of arterial thrombosis are to be balanced against individual bleeding risks. Clinical experience has shown that clopidogrel is to be discontinued ideally approx. 8 to 10 days before vascular surgery on account of increased bleeding risks and that ASA treatment is to be continued. However, cardiovascular risks, e.g., recent implantations of drug-eluting coronary stents, should be considered.
A randomized prospective investigation of dual platelet inhibition vs. ASA monotherapy in 108 patients with CLI and vascular interventions (bypass surgery, major amputation) investigated the course of cardiovascular biomarkers as indicators of coronary events. No significant difference was identified between the patient groups, while the rate of major bleeding was not significantly increased in the group under dual treatment [402].
Following venous bypass setting or synthetic bypass placing below the knee, the Clopidogrel and Acetylsalicylic Acid in Bypass Surgery for Peripheral arterial disease (CASPAR) study randomized 851 subjects to receive ASA (75 to 100 mg) or combined clopidogrel (75 mg) and ASA. No significant difference was seen in patency rates. A subgroup analysis, however, yielded a significant benefit of combination treatment in synthetic over venous bypasses [400].
Based on the results of the CASPAR trial, dual platelet inhibition with ASA and clopidogrel can be considered following infragenual prosthetic bypass surgery.
8.2.2 Heparins
UFH is traditionally given intravenously before applying vascular clamps and discontinuing blood flow in an attempt to prevent thrombosis caused by stasis in the proximal and distal vascular segments, as well as in the anastomotic segment. No randomized studies have addressed this indication and have probably lost their legitimacy due to long-term experience.
General recommendations for intraoperative monitoring of anticoagulation, e.g., based on activated coagulation time (ACT), are not possible because of little available data. As a rule, 100 to 150 U/kg is given intravenously before clamping. Due to the short half-life of UFH (50 to 80 minutes), 50 U/kg is subsequently to be given every 45 to 50 minutes until the vascular clamps are removed and circulation is reestablished [199].
One single study compared three months of postoperative low-molecular-weight heparin (dalteparin 1 × 2,500 U s.c.) with combined ASA and dipyridamole in 200 patients with synthetic bypasses or venous bypasses [387]. The rate of bypass patency was better in the dalteparin group (79 %) than in the group given antiplatelet treatment (64.1 %). A subgroup analysis resulted in significant benefits arising from low-molecular-weight heparin among subjects presenting with CLI as against those with intermittent claudication. This outcome still requires substantiation in a larger patient trial before evidence-based recommendations can be made as to long-term postoperative low-molecular-weight heparin in patients with CLI. In this situation, the additional pleiotropic effects of low-molecular-weight heparins are possibly beneficial.
8.2.3 Postoperative use of VKA
In 1979, a randomized study was carried out in 91 patients with venous femoropopliteal bypass grafts and in 122 patients following thromboendarterectomy. The subjects received ASA (1,000 mg), an ASA-dipyridamole combination, or monotherapy with OACs [403]. There were no significant differences in 2-year patency rates. However, a subgroup analysis revealed improved rates of patency following venous bypass application in the group under OACs. In turn, patients given thrombendarterectomy did better with antiplatelet treatment.
Following placement of venous or synthetic bypasses, 2,690 participants were randomized within the Dutch Bypass Oral Anticoagulants or Aspirin (BOA) study to receive OACs (international normalized ratio [INR] 3 to 4.5) or ASA 80 mg/d [404]. Overall, no benefit was identified in either antithrombotic treatment group as to femoropopliteal vs. femorocrural bypass patency (RR 0.95; 95 % CI 0.82–1.11). Expenditure gains in years with quality of life, and event-free survival were identical with ASA and OAC (INR 3 to 4.5). However, a post-hoc subgroup analysis of bypass material showed a significantly lowered occlusion risk among patients with venous bypasses under OACs, whereas the risks of synthetic bypass occlusions under ASA were significantly lower. Patients under OAC treatment showed significantly more frequent larger-scale bleeding episodes compared with patients under ASA treatment (HR 1.96; 95 % CI 1.42–2.71). Even in the presence of appropriate INR values, study participants above the age of 72 presenting with diabetes and/or arterial hypertension experienced increased bleeding risks.
The results of the BOA trial post-hoc analysis of the patient subgroup with venous grafts is often used as an argument in favor of OAC following venous bypass. According to the evidence criteria, however, post-hoc analyses do not yield sufficient validity to facilitate general recommendations. Moreover, an INR target range of 3 to 4.5 was established for the patient group treated with OACs.
Overall, 2,119 femoropopliteal and 531 femorocrural interventions were performed. The proportionately low number of crural interventions in the study and the high INR target range, which is only recommended for patients at a high risk for thromboembolism [1, 405], together make it difficult to translate these results to the clinical setting. There is insufficient evidence for the currently frequent recommendation of OAC with an INR target range of 2 to 3 following infrapopliteal vascular surgery. Trials addressing this issue are lacking, notwithstanding the small study published by Sarac with 56 patients [406]. With a mid-level OAC dosage (INR 2 to 3) in combination with ASA (325 mg/d), this study documented better bypass patency rates in 56 patients at risk of occlusions (CLI, relapse intervention, poor run-off, poor vein material) as against ASA monotherapy. This applied over a course of 3 years [406].
Additional analysis of the PAD subgroup (stable PAD, CAD with asymptomatic PAD and stable carotid stenosis patients) in the COMPASS trial population [212], as mentioned earlier (see Chapter 6), revealed, besides the 28 % general survival benefit, an additional significant 48 % reduction in major adverse limb events including 70 % major amputation (HR 0.54 95 % CI 0.35–0.82) for ASA 100 mg/d combined with Rivaroxaban 2 × 2.5 mg /d compared to ASS 100 mg/d and placebo. The net benefit was 28 % risk reduction for the PAD subgroup of COMPASS compared to the 24 % MACE reduction in the CAPRIE subgroup [203].
A systematic review concerning the issue of antiplatelet treatment vs. anticoagulation following peripheral vascular bypass identified no advantage of either medical method [401]. The Veterans Affairs trial randomized 831 patients with venous and synthetic bypasses to either low-dose OAC (INR 1.4 to 2.8) and ASA (325 mg) or ASA alone [407]. At a 3-year follow-up, the groups showed no significant differences. This result corresponds to the outcomes of the Warfarin Antiplatelet Vascular Evaluation (WAVE) trial in non-operated high-risk patients with PAD who failed to show benefit under combined ASA and warfarin or ASA alone [408]. Bleeding complications were significantly more frequent, and the risk of bleeding doubled under combined treatment. The subgroup of patients with synthetic bypasses showed a 38 % reduction of risks for bypass occlusions under the combination compared to ASA monotherapy, but accepting significantly increased bleeding rates under combined treatment.
The most recent investigation into this issue compared combined VKA (INR 2.0 to 2.5) plus clopidogrel 75 mg with dual platelet inhibition (clopidogrel 75 mg plus ASA 100 mg) following femoropopliteal bypass surgery up to 9 years of follow-up. Median follow-up was 6.6 years. The participants were either patients with synthetic prostheses above the knee or patients with venous bypasses below the knee. The rate of primary prosthesis patency and number of severe peripheral ischemic events was shown to be higher in the VKA group than in the ASA plus clopidogrel group (p = 0.026 and p = 0.044, respectively). After three years, 97 % and 92 % of the subjects given VKA plus clopidogrel vs. those given ASA plus clopidogrel were amputation-free. The figures were 78 % vs. 64 % of patients after 8 years. The incidence of severe anticoagulation-related complications and overall number of bleeds was seen to be comparable in both groups (p = 0.06 and p = 0.7, respectively), yet the rate of minor bleeding complications was significantly higher in the VKA group (2.85 % per patient-year vs. 1.37 % per patient-year, p = 0.03) [409].
This recommendation is based on the overall analysis of BOA trial data [404] and on the results published by Monaco et al [409].
This recommendation is based on the post-hoc analysis of the BOA trial which demonstrated significantly better patency rates with venous bypasses under VKA (HR: 0.69). In infrainguinal venous bypasses, the benefits were seen regardless of the localization of the distal anastomosis (popliteal, crural, pedal). The ongoing trials with ASA and novel OACs after PTA or vascular surgery [215] will answer the question whether these new drugs offer a safer alternative in this indication.
Low-dose VKA (INR 1.4 to 2.7) combined with ASA (325 mg/d) offers no benefit compared with ASA alone [407]. In this connection, as well, significantly increased rates of bleeding complications were identified. The target range of anticoagulation is to be determined individually following a careful assessment of the risk-benefit ratio, the more so as the target ranges applied to the participants in the Dutch BOA trial were clearly higher than those usually applied in many EU countries.
The extent to which low-molecular-weight heparins may constitute an effective alternative to prevent bypass occlusions in the presence of contraindications against VKA has not been investigated in this patient group.
Patients under anticoagulation and under combination treatment with ASA and VKA are to be closely monitored on account of their clearly increased bleeding risks. This applies particularly to patients under temporary ‘triple’ treatment for cardiac indications, necessitating laboratory tests and clinical control.
The ongoing trials with ASA and NOACs after PTA or vascular surgery [215]. will answer the question whether these new drugs offer a safer alternative in this indication.
8.2.4 Statins
Statins are established secondary prophylactic measures in cardiovascular risk patients, including those with PAD. A multivariate regression analysis of a prospective registry of patients after endovascular interventions showed the combined endpoint of cardiac death, MI, major amputation and re-PTA after statin administration to be significantly reduced over at least 6 to 12 months. Short administration over 30 to 90 days was shown to have no effect [410].
Another large-scale investigation in patients undergoing vascular surgery demonstrated that statin administration may improve the patency rates of vascular bypasses and clinical outcome [411]. A population-based analysis of Medicare data confirmed the positive effect of statin treatment following peripheral vascular interventions [412].
8.3 Follow-up care after vascular interventions
Data are very sparse with regard to the issue of controlled follow-up care after interventional or surgical treatment of peripheral vessels, however across the spectrum of PAD reducing vascular risk is key and should be employed.
A Dutch observational trial in 711 patients consecutively followed over three years following peripheral bypass surgery found 50 % of patients to be given guideline-directed medical treatment with ASA, statins and beta blockers (in patients with ischemic heart disease). After adjusting for clinical characteristics, such treatment was associated with reduced mortality and a hazard ratio of 0.65 (95 % CI 0.45–0.94) [413]. However, based on prescriptions issued 6 to 10 months following primary vascular surgery, a Danish investigation identified low to moderate levels of guideline adherence in terms of lipid-lowering treatments (50 %), ACE inhibition / angiotensin II type 1A (AT1-A) (43 %), and antihypertensive treatment (45 %), as well as a further decrease between 6 months and 3 years post-surgery [414].
In terms of follow-up for patency there are no large-scale controlled randomized trials or systematic overviews that would address the frequency, duration of follow-up, or the method of technical diagnosis following vascular and especially endovascular interventions. However, the restenosis and re-occlusion rates shown in studies in patients with preceding vascular surgery [415, 416] clearly underscore the importance of regular follow-up care and postoperative / post-interventional control. Regular follow-up care is useful, as suggested by results in patency rates following interventional treatment or vascular surgery. According to TASC II, patients with lower-limb bypass surgery due to claudication or CLI are to be admitted to clinical monitoring programs [7]. The postoperative monitoring recommendations contained in the follow-up program of the TASC II guidelines include medical histories, symptom inquiries, clinical examinations and pulse assessment, and ABI/TBI measurements at rest and after exercise for at least two years in the immediate postoperative phase (mostly every 6 months) [7].
Current international guidelines recommend all vascular patients should undergo regular clinical follow-up following peripheral vascular interventions [28, 417]. In one trial, regular follow-up based on duplex sonography was initially recommended for one year following infrainguinal bypass application [415]. The objective of ultrasound examination is to detect possible bypass stenosis (i.a. anastomotic stenosis) early enough to perform (surgical or interventional) reconstructions and avoid bypass occlusion. However, another randomized multicenter study failed to substantiate the benefits and cost effectiveness of such routine ultrasound examinations [418]. Over 18 months, regular duplex sonography applied to control venous bypasses yielded neither an increase in secondary patency rates nor improvements in patients’ quality of life.
Despite these divergent study results and evidence lack, many vascular medicine centers have well established postoperative and post-interventional monitoring of vascular reconstruction. For the reasons stated above, however, no generally valid standards have yet been formulated, though many experts agree an annual review. Analogous to the results of the BASIL trial and many other clinical studies following revascularization, secondary patency rates are the consequence of regularly performed examinations. This applies equally to surgical and interventional treatments.
It remains unclear whether duplex monitoring or clinical examinations are better and more cost-effective. In general, measurement of peripheral occlusive pressure following successful arterial revascularization, ABI/TBI assessment, and clinical physical examinations including pedal pulse palpation are recommended. In patients with diabetes, these measures are recommended to be on a yearly basis in the framework of disease management programs.
To account for the significance of cardiovascular risk factors and comorbidity (particularly CAD and cerebral circulation disorders), all patients with detected PAD should, where possible, be integrated in a registry program. This program should include vascular risk factors, and their management, in addition to the symptoms and diagnosis of PAD.
8.3.1 Follow-up vascular training/exercise
Following endovascular treatment in the aortoiliac segment, and albeit based on small patient numbers, the results of several trials showed significant improvements in maximum walking distances with subsequent structured vascular training compared to no subsequent vascular exercise [233]. Only one investigation has evidenced improvements in walking distances due to additive walking exercise following surgical vascular reconstruction. This randomized controlled trial showed that, even after 6 months, additional walking training after peripheral bypass surgery resulted in significantly longer walking distances and improved quality of life in the interventional group compared to the control group [419]. No effect was seen in a less recent and smaller randomized trial. Overall, however, the available results indicate lack of exercise to be a risk factor for vascular occlusions following surgical vascular reconstruction.
For this reason, regular physical exercise appears useful. It also seems possible, although not yet investigated, that this observation may lead to reduced rates of restenosis and re-occlusion after PTA for patients having undergone interventional treatment. Autonomous and daily interval training over 60 minutes with 5- to 15-minute effort intervals is recommended, where intensity is to reach maximal pain [227, 228]. Nordic walking may be recommended as a variation (the positive metabolic effect is higher but the workload for the legs is lower than without walking sticks).
8.4 Rehabilitation in PAD
Treating physicians are responsible for giving patients information about the options of post-intervention rehabilitation. Clinicians may initiate inpatient or outpatient care in the form of follow-up rehabilitation. Follow-up rehabilitation should begin within 14 days after discharge from acute treatment.
As rehabilitation measures are indicated, physicians should consider the following issues:
- •Is the given rehabilitation measure indicated, i.e. is participation impaired and is there a (participation) objective to be reached with rehabilitation?
- •Is the given patient capable of being rehabilitated, i.e. can he/she participate in rehabilitative treatment?
- •What is the prognosis of rehabilitation outcome, i.e. is there a realistic chance that the patient will meet the intended rehabilitation objective?
These measures aim at the (partial) recovery of organ function. In many cases, the main task of rehabilitation is to exploit the possibilities of compensation in an attempt to replace damaged organ functions. The less completely a medical rehabilitation is achieved, the more the patient’s personal and family behavior and environment must adjust.
Rehabilitation consists of the following steps
The individual objectives of rehabilitation are dependent upon the patients’ extent of occupational and social participation.
- •The rehabilitation process covers medical treatment, physical therapy and ergotherapy, exercise treatment adapted to the given patient’s level of performance, disorder-specific courses of instruction and on nutrition, which are altogether coordinated within an interdisciplinary rehabilitation team under a physician’s guidance. As necessary, this treatment plan also includes stage-adapted wound management.
- •Final assessments serve to determine whether the objectives of rehabilitation have been met, to formulate recommendations for further treatment, and to issue sociomedical assessments of the patients’ performance levels.
Rehabilitation can be carried out on an outpatient or inpatient basis. Inpatient rehabilitation is to be offered as needed. Patients with PAD are not to be refused rehabilitation merely because adequate and accessible outpatient institutions are not present. The lack of outpatient institutions providing angiology rehabilitation and vascular sports groups is striking. It remains a joint challenge for funding agencies, societies of vascular medicine and patients’ support groups to find a remedy in this connection.
8.5 Post-amputation follow-up care
Patients’ occupational and social rehabilitation are to be initiated along with their medical rehabilitation. Adequate and individually fitted footgear is recommended following minor amputations. Procedures following amputations consist of wound and stump management, contracture prophylaxis, remedial gymnastics, and muscle-building training.
Major amputations entail secondary wound management and treatment for possible phantom limb pain. Stump pain is to be distinguished from phantom pain which is reduced with timely epidural regional anaesthesia [420] as long as there are no contraindications. Interdisciplinary treatment is often successful in persistent pain conditions.
Psychological support with an aim to overcome amputation, is a critical constituent. Temporary prostheses are to be indicated for all amputees, especially if the objective is mobilisation via prosthesis from bed into a wheelchair. If suitable, patients are to be offered prostheses training with standing and walking exercises, as well as gait and walking training.
In terms of stabilized mobilization and the secondary prevention of further cardiovascular events, one of the targets is participation in para-sports and rehabilitation near outpatient centers connected to vascular offices and clinics. The aim of treatment, following high-level amputations, is participation in a self-determined and fulfilled life, as well as social reintegration. This comprises of consultation and support: home care, disability-friendly living, support in prosthesis care, disabled persons’ passes, nursing care insurances, retraining, shuttle services, motor-vehicle conversions, and as much as networking with medical specialists in specialty hospitals, physical therapists and podiatrists as is required.
9 The elderly patient with PAD
Multi-morbidity of old age comprises the following characteristics: Immobility, a tendency to fall with/without vertigo, cognitive deficits including dementia, incontinence, decubitus ulcers, malnutrition and nutritional deficiency, fluid-balance disorders and electrolyte imbalance, depression and anxiety disorders, chronic pain syndrome, sensory disturbances, visual and hearing impairment, and medication problems. The concept of “frailty syndrome” and “reduced resilience” can also be used to describe this constellation of symptoms. Furthermore, multiple and often simultaneous organ-specific disorders tend to present atypically.
Reduced ability to compensate for different stressors, including diseases, social changes and bereavement, is typical of the very elderly with multi-morbidity problems. It manifests as increased risk of disease complications and treatment adverse events, prolonged convalescence, together with a high risk associated with experiencing a loss of independence. Therefore, both the prevention of avoidable morbidity and targeted rehabilitative follow-up in such a group play a critical role in patient management and should be considered in formulating treatment guidelines.
The prevalence of PAD increases with age and reaches over 15–20 % among subjects beyond the age of 70 [9, 10]; and the proportion in diabetic patients then in people without diabetes [56]. Qualitatively comparable epidemiological studies in the frail elderly patient are lacking. However, the frequency of PAD is assumed to be even higher in this patient group. For example, the prevalence of symptomatic PAD was seen to total 32 % (men) and 26 % (women) among 3,500 care home occupants aged 81 on average [421].
Among fit elderly patients, the clinical presentation of PAD is marked by a more frequent presentation of CLI versus claudication, especially among patients with diabetes [422]. There are indications also that advanced, yet clinically silent PAD is even higher among frail elderly patients [423]. Elderly subjects (both men and women) presenting with subclinical PAD have already been shown to suffer from mobility impairment [424]. Walking sticks or rollators become frequently required in elderly PAD patients due to ischemic muscle atrophy, inability to control their legs and sensory impairments, often leading to a higher risk of falling with resulting injuries including fractures. Detection of PAD in frail elderly patients is thus significant in terms of prevention: Early revascularization may prevent such walking impairment. Furthermore, the risk of pressure lesions e.g. on the heel are particularly increased in the bed-bound patient. Conversely, undetected PAD following foot or lower-leg interventions may contribute to wound healing problems [425].
Once PAD has become symptomatic in elderly patients, it often manifests in pedal/crural trophic lesions or in pain at rest. Moreover, frail elderly subjects often experience simultaneous coronary artery disease (68 %) or stroke (42 %) [426]. Generally, increased treatment risks are associated with such multi-morbid patients. A small number of retrospective studies have explicitly addressed PAD treatment in elderly patients. The trait of frailty is consistently described as detrimental in terms of maintaining self-sufficiency following vascular surgery or catheter-based interventions for critical limb ischemia [427]. The risks of confusion, falls, decubitus ulcers and loss of autonomy are significantly higher with emergency surgery than elective interventions, particularly in the presence of pre-existing functional limitations (activities of daily living, ADL) [428]. Despite this, actual vascular results are good with the rate of leg salvage following successful vascular interventions being reported as high as approximately 90 % [429].
The incidence of postoperative confusion in patients with arterial vascular reconstructions is approximately 40 % which is a further argument for the PTA-first strategy in old and very old PAD patients. Pre-existing dementia, critical leg ischemia and age above 72 years are independent risk factors for the development of postoperative confusion in patients with CLI [430].
The basic principles in the diagnosis and treatment of PAD apply also to frail elderly patients. The recommendations given in various parts of the present guidelines are thus to be considered in this patient group, especially those referring to the risk-benefit assessments of diagnostic and therapeutic measures. It is crucial to understand the extent to which symptoms of PAD influence frail elderly patients’ reduced overall quality of life, and balance them in relation to the invasiveness of PAD treatments.
Frail elderly patients frequently experience lengthy periods of convalescence with initial reduced rehabilitative abilities due to acute medical complications and problems in recovering accustomed degrees of independence (e.g., because of transient disorientation, incontinence, frailty syndrome). This is the target patient group of early geriatric rehabilitation which is performed either in specialty hospitals or hospital divisions of geriatrics/medicine for the elderly with high rehabilitative-geriatric quality standards. Alternatively, it can be provided by geriatric rehabilitation institutions meeting the staff and technical requirements that are necessary to carry out such early geriatric rehabilitation to high acute medical standards. Initiation of comprehensive geriatric rehabilitation should be considered if circulatory stability and overall health allow active participation in rehabilitative programs several times a day. Geriatric rehabilitation has been proven to lower mortality, improve functional outcomes, and reduce the number of referrals to care homes [431].
References
1 S3-Leitlinie zur Diagnostik, Therapie und Nachsorge der peripheren arteriellen Verschlusskrankheit. 2015.
2 . Recommendations for Guideline Production. https://wwwescardioorg/static_file/Escardio/Guidelines/ESC%20Guidelines%20for%20Guidelines%20Update%202010pdf. 2010.
3 ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463–654.
4 . Multi-bed vascular disease and atherothrombosis: scope of the problem. J Thromb Thrombolysis. 2004;17(1):51–61.
5 Diagnostic value of ankle-brachial index in peripheral arterial disease: a meta-analysis. Can J Cardiol. 2013;29(4):492–8.
6 Evaluation of the one-minute exercise test to detect peripheral arterial disease. European Journal of Clinical Investigation. 2008;38(5):290–5.
7 Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33(Suppl 1):S1–75.
8 . The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71(3):510–5.
9 High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis. 2004;172(1):95–105.
10 Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–40.
11 . Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1991;20(2):384–92.
12 . 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS)Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries Endorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). European Heart Journal. 2018;39(9):763–816.
13 Heart Failure Risk Across the Spectrum of Ankle-Brachial Index: The ARIC Study (Atherosclerosis Risk In Communities). Heart Failure. 2014;2(5):447–54.
14 Comparison of two coronary risk equivalents: diabetes mellitus and peripheral arterial disease. Dtsch Med Wochenschr. 2008;133(45):2317–22.
15 Prevalence of peripheral arterial disease – results of the Heinz Nixdorf recall study. Eur J Epidemiol. 2006;21(4):279–85.
16 Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317–24.
17 The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337.
18 A systematic review for the screening for peripheral arterial disease in asymptomatic patients. J Vasc Surg. 2015;61(3 Suppl):42S–53S.
19 Lessons learned from the analysis of gender effect on risk factors and procedural outcomes of lower extremity arterial disease. J Vasc Surg. 2010;52(5):1196–202.
20 Gender Differences in Outcomes of Endovascular Treatment of Infrainguinal Peripheral Artery Disease. Vascular and Endovascular Surgery. 2011;45(8):703–11.
21 . Gender is an independent risk factor for distribution pattern and lesion morphology in chronic critical limb ischemia. J Vasc Surg. 2012;55(1):98–104.
22 Ethnicity and peripheral arterial disease: the San Diego Population Study. Circulation. 2005;112(17):2703–7.
23 . Ethnic differences in peripheral arterial disease in the NHLBI Genetic Epidemiology Network of Arteriopathy (GENOA) study. Vasc Med. 2003;8(4):237–42.
24 . Risk factors for atherosclerotic cardiovascular outcomes in different arterial territories. J Cardiovasc Risk. 1994;1(4):333–9.
25 . Vascular event rates in patients with atherosclerotic cerebrovascular disease. Arch Neurol. 1992;49(8):857–63.
26 . Second Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II). Circulation. 1994;89(3):1333–445.
27 Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326(6):381–6.
28 ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2011;32(22):2851–906.
29 Prevalence of unknown peripheral arterial disease in patients with coronary artery disease: data in primary care from the IPSILON study. Arch Cardiovasc Dis. 2009;102(8–9):625–31.
30 Prediction of coronary artery disease in patients with lower extremity peripheral artery disease. Int Heart J. 2015;56(2):209–12.
31 One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297(11):1197–206.
32 Intermittent claudication as a predictor of outcome in patients with ischaemic systolic heart failure: analysis of the Controlled Rosuvastatin Multinational Trial in Heart Failure trial (CORONA). European Journal of Heart Failure. 2010. [cited 12 7]; 698–705. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1093/eurjhf/hfq070
33 Effect of peripheral arterial disease on functional and clinical outcomes in patients with heart failure (from HF-ACTION). Am J Cardiol. 2011;108(3):380–4.
34 Heart failure is associated with reduced patency after endovascular intervention for symptomatic peripheral arterial disease. J Vasc Surg. 2012;55(2):353–62.
35 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD – summary. Diab Vasc Dis Res. 2014;11(3):133–73.
36 . Glycosylated hemoglobin in relationship to cardiovascular outcomes and death in patients with type 2 diabetes: a systematic review and meta-analysis. PLoS One. 2012;7(8):e42551.
37 . Prevalence of neuropathy and peripheral arterial disease and the impact of treatment in people with screen-detected type 2 diabetes: the ADDITION-Denmark study. Diabetes Care. 2011;34(10):2244–9.
38 Typ-2-Diabetes. Präventions- und Behandlungsstrategien für Fußkomplikationen. AWMF. 2010;2.8.
39 . Prevalence and Risk Factors of PAD among Patients with Elevated ABI. European Journal of Vascular and Endovascular Surgery. 2008;35(6):709–14.
40 Critical limb ischaemia: initial treatment and predictors of amputation-free survival. Eur J Vasc Endovasc Surg. 2012;43(1):55–61.
41 . Stroke and chronic kidney disease: epidemiology, pathogenesis, and management across kidney disease stages. Seminars in Nephrology. 2015;35(4):311–22.
42 . Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86.
43 Different calculations of ankle-brachial index and their impact on cardiovascular risk prediction. Circulation. 2008;118(9):961–7.
44 . Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114(7):688–99.
45 Association of low ankle brachial index with high mortality in primary care. Eur Heart J. 2006;27(14):1743–9.
46 . Defining the outcome of critical ischemia: a one year prospective study. Br J Surg. 1986;73:321.
47 . Characteristics and Outcome of Patients Hospitalised for Lower Extremity Peripheral Artery Disease in France: The COPART Registry. European Journal of Vascular and Endovascular Surgery. 2010;39(5):577–85.
48 Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. The Lancet. 2005;366(9501):1925–34.
49 . The risk of disease progression in peripheral arterial disease is higher than expected: a meta-analysis of mortality and disease progression in peripheral arterial disease. European Journal of Vascular and Endovascular Surgery. 2016;51(3):395–403.
50 . Outcome and quality of life of patients with severe chronic limb ischaemia: A cohort study on the influence of diabetes. European Journal of Vascular and Endovascular Surgery. 1995;10(4):459–65.
51 . The Scottish Diabetes Foot Action Group 2016 update of the Diabetic Foot Risk Stratification and Triage System. Wounds International. 2017;8(1):25–8.
52 Recent trends in morbidity and in-hospital outcomes of in-patients with peripheral arterial disease: a nationwide population-based analysis. Eur Heart J. 2013;34(34):2706–14.
53 Peripheral arterial disease and critical limb ischaemia: still poor outcomes and lack of guideline adherence. European Heart Journal. 2015;36(15):932–8.
54 . The compelling arguments for the need of medical vascular physicians in Europe. Vasa. 2019;4:1–5.
55 Prävalenz, Komorbidität und Behandlungsintensität der peripheren arteriellen Verschlusskrankheit in der Hausarztpraxis. Gefässchirurgie. 2004;9(3):166–71.
56 High prevalence of peripheral arterial disease and low treatment rates in elderly primary care patients with diabetes. Exp Clin Endocrinol Diabetes. 2004;112(10):566–73.
57 International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295(2):180–9.
58 Gaps in public knowledge of peripheral arterial disease: the first national PAD public awareness survey. Circulation. 2007;116(18):2086–94.
59 Contemporary cardiovascular risk and secondary preventive drug treatment patterns in peripheral artery disease patients undergoing revascularization. Journal of Vascular Surgery. 2016;64(4):1009–17.e3.
60 Does the clinical examination predict lower extremity peripheral arterial disease? Jama. 2006;295(5):536–46.
61 . Occlusive peripheral artery disease. Early diagnosis, incidence, course, significance. Hans Huber, Bern, Stuttgart, Wien. 1980;1–97.
62 . Guidelines for the diagnosis and outpatient management of diabetic peripheral neuropathy. Diabet Med. 1998;15(6):508–14.
63 . Preventing amputation in the patient with diabetes. Diabetes Care. 1995;18(10):1383–94.
64 Kuopio Atherosclerosis Prevention Study (KAPS). A population-based primary preventive trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteries. Circulation. 1995;92(7):1758–64.
65 . Un nuovo sfigmomanometro. Gazzetta medica di Torino. 1896;47:981–1001.
66 Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126(24):2890–909.
67 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (Updating the 2005 Guideline). Journal of the American College of Cardiology. 2011;58(19):2020–45.
68 . Diagnosis and management of lower limb peripheral arterial disease: summary of NICE guidance. BMJ: British Medical Journal. 2012:345.
69 Reproducibility and reliability of the ankle-brachial index as assessed by vascular experts, family physicians and nurses. Vasc Med. 2007;12(2):105–12.
70 . Noninvasive vascular laboratory for evaluation of peripheral arterial occlusive disease: Part II – clinical applications: chronic, usually atherosclerotic, lower extremity ischemia. J Vasc Interv Radiol. 2000;11(10):1257–75.
71 . Plantar flexion as an alternative to treadmill exercise for evaluating patients with intermittent claudication. European Journal of Vascular and Endovascular Surgery. 2007;33(3):325–9.
72 . The walking impairment questionnaire: An effective tool to assess the effect of treatment in patients with intermittent claudication. Journal of Vascular Surgery. 2009;50(1):89–94.
73 . The association between elevated ankle systolic pressures and peripheral occlusive arterial disease in diabetic and nondiabetic subjects. J Vasc Surg. 2008;48(5):1197–203.
74 . Falsely elevated ankle pressures in severe leg ischaemia: the pole test – an alternative approach. Eur J Vasc Surg. 1994;8(4):408–12.
75 . Ankle-brachial index, toe-brachial index, and cardiovascular mortality in persons with and without diabetes mellitus. J Vasc Surg. 2014;60(2):390–5.
76 . Hide and seek: does the toe-brachial index allow for earlier recognition of peripheral arterial disease in diabetic patients? Eur J Vasc Endovasc Surg. 2015;49(2):192–8.
77 . The toe-brachial index in the diagnosis of peripheral arterial disease. J Vasc Surg. 2013;58(1):231–8.
78 . Value of the duplex waveform at the common femoral artery for diagnosing obstructive aortoiliac disease. J Vasc Surg. 2005;42(2):236–42.; discussion 42.
79 . The use of transcutaneous oximetry to predict complications of chronic wound healing: a systematic review and meta-analysis. Wound Repair Regen. 2011;19(6):657–63.
80 . Evaluation of clinical tests to assess perfusion in chronic critical limb ischemia. Vasa. 2002;31(3):173–8.
81 Selection of patients with lower limb arterial occlusive disease for endovascular treatment of the iliac arteries with duplex scanning. Vasc Surg. 2001;35(6):437–42.
82 Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: systematic review. BMJ. 2007;334(7606):1257.
83 Duplex sonography versus angiography for assessment of femoropopliteal arterial disease in a “real-world” setting. J Endovasc Ther. 2007;14(4):452–9.
84 Effect of amlodipine on the progression of atherosclerosis and the occurrence of clinical events. PREVENT Investigators. Circulation. 2000;102(13):1503–10.
85 . Duplex scanning of the peripheral arteries: correlation of the peak velocity ratio with angiographic diameter reduction. Ultrasound Med Biol. 1992;18(5):433–40.
86 . Vessel wall calcifications at multi-detector row CT angiography in patients with peripheral arterial disease: effect on clinical utility and clinical predictors. Radiology. 2006;241(2):603–8.
87 Agreement of duplex ultrasonography vs. computed tomography angiography for evaluation of native and in-stent SFA re-stenosis –findings from a randomized controlled trial. Eur J Radiol. 2012;81(9):2265–9.
88 . Diagnostic performance of computed tomography angiography in peripheral arterial disease: A systematic review and meta-analysis. JAMA. 2009;301(4):415–24.
89 Contrast-enhanced magnetic resonance angiography in diabetic patients with infra-genicular peripheral arterial disease: systematic review. Int J Surg. 2013;11(3):228–32.
90 . Nephrogenic systemic fibrosis is not exclusively associated with gadodiamide. European Radiology. 2007;17(8):1921–3.
91 . Preventing nephropathy induced by contrast medium. New England Journal of Medicine. 2006;354(4):379–86.
92 . The clinical epidemiology of contrast-induced nephropathy. Cardiovasc Intervent Radiol. 2005;28(Suppl 2):S3–11.
93 . Iso-osmolality versus low-osmolality iodinated contrast medium at intravenous contrast-enhanced CT: effect on kidney function. Radiology. 2008;248(1):97–105.
94 . Radiocontrast-induced acute renal failure. J Intensive Care Med. 2005;20(2):63–75.
95 . Determination of serum creatinine level before intravenous administration of iodinated contrast medium. A survey. Invest Radiol. 1995;30(12):700–5.
96 , National Kidney Foundation Primer on Kidney Diseases. (7th ed.): Elsevier; 2018.
97 . Updated contrast-induced nephropathy (CIN) prevention guidelines 2014. ESC. 2014;13(4).
98 Outcomes after angiography with sodium bicarbonate and acetylcysteine. New England Journal of Medicine. 2017;378(7):603–14.
99 . Perchlorate and the thyroid gland. Pharmacological Reviews. 1998;50(1):89–106.
100 . Carbon dioxide angiography is a standard technique to supplement iodinated contrast angiography and can be a feasible alternative. Angiology. 2016;67(10):974.
101 . Importance of ultrasound for diagnosing peripheral arterial disease. Ultraschall Med. 2009;30(4):334–74.
102 . Diabetes care for patients with peripheral arterial disease. Eur J Vasc Endovasc Surg. 2007;33(5):583–91.
103 . Infrainguinal revascularization because of claudication: total long-term outcome of endovascular and surgical treatment. J Vasc Surg. 2003;37(4):808–15.
104 . Changing pattern of surgical revascularization for critical limb ischemia over 12 years: endovascular vs. open bypass surgery. J Vasc Surg. 2006;44(2):304–13.
105 Early surgical outcome after failed primary stenting for lower limb occlusive disease. J Endovasc Ther. 2005;12(1):13–21.
106 . Impact of increasing comorbidity on infrainguinal reconstruction: a 20-year perspective. Ann Surg. 2001;233(3):445–52.
107 . Angiographic distribution of lower extremity atherosclerosis in patients with and without diabetes. Diabet Med. 2002;19(5):366–70.
108 . Early and late outcomes of percutaneous treatment of TransAtlantic Inter-Society Consensus class C and D aorto-iliac lesions. Journal of Vascular Surgery. 2011;53(6):1728–37.
109 . Tibioperoneal (outflow lesion) angioplasty can be used as primary treatment in 235 patients with critical limb ischemia: five-year follow-up. Circulation. 2001;104(17):2057–62.
110 . A systematic review of endovascular treatment of extensive aortoiliac occlusive disease. Journal of Vascular Surgery. 2010;52(5):1376–83.
111 . Decreasing incidence of major amputations in people with diabetes. Diabetologia. 2000;43(7):844–7.
112 Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: A survival prediction model to facilitate clinical decision making. Journal of Vascular Surgery. 2010;51(5, Supplement):52S–68S.
113 ESC/EAS Guidelines for the Management of Dyslipidaemias. European Heart Journal. 2016;37(39):2999–3058.
114 . Standards of Medical Care in Diabetes-2019. American Diabetes Association. 2019;42(Supplement 1):S4–S183.
115 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013 Oct;34(39):3035–87.
116 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159–219.
117 Fourth Joint Task Force of the European Society of Cardiology and other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). European Journal of Cardiovascular Prevention & Rehabilitation. 2007;14(2 suppl):S1–S.
118 . The role of tobacco cessation, antiplatelet and lipid-lowering therapies in the treatment of peripheral arterial disease. Vascular Medicine. 1997;2(3):243–51.
119 . Smoking, hemorheologic factors, and progression of peripheral arterial disease in patients with claudication. Journal of Vascular Surgery. 1998;28(1):129–35.
120 Influence of smoking on incidence and prevalence of peripheral arterial disease. Journal of Vascular Surgery. 2004;40(6):1158–65.
121 . The cardiologist and smoking cessation. Current Opinion in Cardiology. 2010;25(5):469–77.
122 A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. New England Journal of Medicine. 1999;340(9):685–91.
123 Triple-combination pharmacotherapy for medically ill smokers: a randomized trial. Ann Intern Med. 2009;150(7):447–54.
124 Effectiveness of a smoking cessation program for peripheral artery disease patients. A Randomized Controlled Trial. 2010;56(25):2105–12.
125 . Association of anger proneness, depression and low social support with peripheral arterial disease: the Atherosclerosis Risk in Communities Study. Vascular Medicine. 2005;10(3):199–206.
126 . Twenty-year predictors of peripheral arterial disease compared with coronary heart disease in the Scottish heart health extended cohort (SHHEC). Journal of the American Heart Association. 2017;6(9):e005967.
127 Association between ankle – brachial index and risk factor profile in patients newly diagnosed with intermittent claudication. Circulation Journal. 2008;72(3):441–8.
128 . Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet (London, England). 2003;361(9374):2005–16.
129 . MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebocontrolled trial. The Lancet. 2002;360(9326):7–22.
130 . Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg. 2007;45(4):645–54;discussion 53–4.
131 Statins for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews. 2013(1).
132 Evolocumab and clinical outcomes in patients with cardiovascular disease. New England Journal of Medicine. 2017;376(18):1713–22.
133 Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER trial (further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk). Circulation. 2018;137(4):338–50.
134 Effect of simvastatin on ischemic signs and symptoms in the Scandinavian simvastatin survival study (4S). The American Journal of Cardiology. 1998;81(3):333–5.
135 . Effect of simvastatin versus placebo on treadmill exercise time until the onset of intermittent claudication in older patients with peripheral arterial disease at six months and at one year after treatment. The American Journal of Cardiology. 2003;92(6):711–2.
136 . Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation. 2003;108(12):1481–6.
137 . Drug therapy for improving walking distance in intermittent claudication: a systematic review and meta-analysis of robust randomised controlled studies. European Journal of Vascular and Endovascular Surgery. 2009;38(4):463–74.
138 Statin use and lower extremity amputation risk in nonelderly diabetic patients. Journal of Vascular Surgery. 2013;58(6):1578–85.e1.
139 . Statin therapy reduces future risk of lower-limb amputation in patients with diabetes and peripheral artery disease. The Journal of Clinical Endocrinology & Metabolism. 2017;102(7):2373–81.
140 Statin therapy is associated with improved patency of autogenous infrainguinal bypass grafts. Journal of Vascular Surgery. 2004;39(6):1178–85.
141 Association of statin dose with amputation and survival in patients with peripheral artery disease. Circulation. 2018;137(14):1435–46.
142 Ezetimibe added to statin therapy after acute coronary syndromes. New England Journal of Medicine. 2015;372(25):2387–97.
143 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014;63(25 Part B):2889–934.
144 . Updated cost-effectiveness analysis of evolocumab in patients with very high-risk atherosclerotic cardiovascular disease. JAMA Cardiology. 2019. 5th June 2019 ed. online.
145 . Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110(23):3512–7.
146 Effect of niacin ER/lovastatin on claudication symptoms in patients with peripheral artery disease. Vasc Med. 2010;15(3):171–9.
147 . Omega-3 dietary supplements and the risk of cardiovascular events: a systematic review. Clin Cardiol. 2009;32(7):365–72.
148 . Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308(10):1024–33.
149 . Effect of Omega-3 fatty acid supplementation on markers of platelet and endothelial function in patients with peripheral arterial disease. Atherosclerosis. 2012;221(2):514–20.
150 n-3 Polyunsaturated fatty acids supplementation in peripheral artery disease: the OMEGA-PAD trial. Vasc Med. 2013;18(5):263–74.
151 . Omega-3 fatty acids for intermittent claudication. Cochrane Database Syst Rev. 2013;4(7):CD003833.
152 . UKPDS 59: Hyperglycemia and other potentially modifiable risk factors for peripheral vascular disease in type 2 diabetes. Diabetes Care. 2002;25(5):894–9.
153 Peripheral artery disease, diabetes, and reduced lower extremity functioning. Diabetes Care. 2002;25(1):113–20.
154 . Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. New England Journal of Medicine. 2003;348(5):383–93.
155 Therapie des Typ 2 Diabetes. Nationale Versorgungsleitlinie. 2013.
156 Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–79.
157 Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59.
158 Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72.
159 . ADA Scientific Session. June; 8, 2008.
160 Follow-up of Blood-Pressure Lowering and Glucose Control in Type 2 Diabetes. New England Journal of Medicine. 2014;371(15):1392–406.
161 Metformin is associated with improved survival and decreased cardiac events with no impact on patency and limb salvage after revascularization for peripheral arterial disease. Ann Vasc Surg. 2019;55:63–77.
162 Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes Metab. 2014;16(11):1165–73.
163 Heart failure, saxagliptin, and diabetes mellitus: Observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2015;132(15):e198.
164 Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327–35.
165 Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385(9982):2067–76.
166 Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373(3):232–42.
167 CAROLINA,®. Cardiovascular safety and renal microvascular outcome with linagliptin in patients with T2D at high vascular risk.
Oral presentation at the 79th Scientific Sessions of the American Diabetes Association (ADA) ,Monday, 10 June 2019, 16:30–18:30 ,San Francisco, CA, USA . 2019.168 Liraglutide and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine. 2016;375(4):311–22.
169 Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375(19):1834–44.
170 Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. The Lancet. 2019;394(10193):121–30.
171 Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. The Lancet. 2005;366(9493):1279–89.
172 . The Effect of Pioglitazone on Recurrent Myocardial Infarction in 2,445 Patients With Type 2 Diabetes and Previous Myocardial Infarction: Results From the PROactive (PROactive 05) Study. Journal of the American College of Cardiology. 2007;49(17):1772–80.
173 Effects of Pioglitazone in Patients With Type 2 Diabetes With or Without Previous Stroke: results From PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04). Stroke. 2007;38(3):865–73.
174 . Pioglitazone and Risk of Cardiovascular Events in Patients With Type 2 Diabetes MellitusA Meta-analysis of Randomized Trials. JAMA. 2007;298(10):1180–8.
175 Pioglitazone after ischemic stroke or transient ischemic attack. New England Journal of Medicine. 2016;374(14):1321–31.
176 Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. New England Journal of Medicine. 2015;373(22):2117–28.
177 Empagliflozin and progression of kidney disease in type 2 diabetes. New England Journal of Medicine. 2016;375(4):323–34.
178 Canagliflozin and cardiovascular and renal events in type 2 diabetes. New England Journal of Medicine. 2017;377(21):2097–9.
179 Dapagliflozin and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine. 2018;380(4):347–57.
180 Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): a randomised, double-blind, placebo-controlled trial. The Lancet Diabetes & Endocrinology. 2017;5(11):877–86.
181 Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367(4):319–28.
182 . Epidemiology of peripheral artery disease. Circulation Research. 2015;116(9):1509–26.
183 Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308(16):1660–7.
184 Usual blood pressure, peripheral arterial disease, and vascular risk: cohort study of 4.2 million adults. BMJ: British Medical Journal. 2015;351:h4865.
185 . Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels – updated overview and meta-analyses of randomized trials. Journal of Hypertension. 2016;34(4):613–22.
186 Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. The Lancet. 2016;387(10022):957–67.
187 Systematic Review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. 2017.
188 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. 2017.
189 2018 ESC/ESH Guidelines for the management of arterial hypertension. European Heart Journal. 2018;39(33):3021–104.
190 Effects of an Angiotensin-Converting–Enzyme Inhibitor, Ramipril, on Cardiovascular Events in High-Risk Patients. New England Journal of Medicine. 2000;342(3):145–53.
191 Telmisartan, Ramipril, or both in patients at high risk for vascular events. New England Journal of Medicine. 2008;358(15):1547–59.
192 Impact of ramipril in patients with evidence of clinical or subclinical peripheral arterial disease. European Heart Journal. 2004;25(1):17–24.
193 . Meta-analysis of angiotensin converting enzyme inhibitors effect on walking ability and ankle brachial pressure index in patients with intermittent claudication. Atherosclerosis. 2013;231(2):283–90.
194 . Angiotensin converting enzyme inhibitors and walking distance: Have we walked the whole distance? Atherosclerosis. 2016;252:199–200.
195 . Effect of verapamil in intermittent claudication. Circulation. 1997;95(2):411–4.
196 Blockers in patients with intermittent claudication and arterial hypertension. Hypertension. 2011;58(2):148–54.
197 . Beta blockers for peripheral arterial disease. Cochrane Database of Systematic Reviews. 2013(9).
198 . Beta-blocker treatment does not worsen critical limb ischemia in patients receiving endovascular therapy. Journal of Atherosclerosis and Thrombosis. 2015;22(5):481–9.
199 , Antithrombotic therapy for peripheral artery occlusive disease: American college of chest physicians evidence-based clinical practice guidelines (8th Edition). Chest. 2008(Supplement 6);133:815S–43S.
200 . Aspirin for the primary prevention of cardiovascular events in patients with peripheral artery disease or diabetes mellitus. Analyses from the JPAD, POPADAD and AAA trials. Thromb Haemost. 2010;104(6):1085–8.
201 . Antithrombotic treatment in peripheral artery disease. Vasa. 2018;47(2):99–108.
202 . Antithrombotic Therapy in Peripheral Arterial Occlusive Disease: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3, Supplement):609S–26S.
203 A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). The Lancet. 1996;348(9038):1329–39.
204 Prevention of myocardial infarction and stroke in patients with intermittent claudication; effects of ticlopidine. Results from STIMS, the Swedish Ticlopidine Multicentre Study. J Intern Med. 1990;227(5):301–8.
205 Ticagrelor compared with clopidogrel in patients with prior lower extremity revascularization for peripheral artery disease. Circulation. 2017;135(3):241–50.
206 Vorapaxar in the secondary prevention of atherothrombotic events. New England Journal of Medicine. 2012;366(15):1404–13.
207 Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. New England Journal of Medicine. 2006;354(16):1706–17.
208 Long-term use of ticagrelor in patients with prior myocardial infarction. New England Journal of Medicine. 2015;372(19):1791–800.
209 Efficacy and safety of ticagrelor as long-term secondary prevention in patients with prior myocardial infarction and peripheral artery disease. Journal of the American College of Cardiology. 2016;67(13 Supplement):2266.
210 Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010;303(9):841–8.
211 Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. New England Journal of Medicine. 2017;377(14):1319–30.
212 Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. The Lancet. 2018;391(10117):219–29.
213 Major adverse limb events and mortality in patients with peripheral artery disease: The COMPASS Trial. Journal of the American College of Cardiology. 2018;71(20):2306–15.
214 Rivaroxaban with or without aspirin in patients with heart failure and chronic coronary or peripheral artery disease. Circulation. 2019;140(7):529–37.
215 Rationale and design for the Vascular Outcomes study of ASA along with rivaroxaban in endovascular or surgical limb revascularization for peripheral artery disease (VOYAGER PAD). Am Heart J. 2018;199:83–91.
216 . Medical management of peripheral arterial disease. J Thromb Haemost. 2005;3(8):1628–37.
217 . Exercise for intermittent claudication. Cochrane Database Syst Rev. 2014;18(7):CD000990.
218 Supervised walking therapy in patients with intermittent claudication. Journal of Vascular Surgery. 2012;56(4):1132–42.
219 . Supervised exercise therapy versus non-supervised exercise therapy for intermittent claudication. Cochrane Database Syst Rev. 2013;23(8):CD005263.
220 . Upper- versus lower-limb aerobic exercise training on health-related quality of life in patients with symptomatic peripheral arterial disease. Journal of Vascular Surgery. 2011;53(5):1265–73.
221 . Modes of exercise training for intermittent claudication. Cochrane Database Syst Rev. 2014;4(7):CD009638.
222 . Superiority of treadmill walking exercise versus strength training for patients with peripheral arterial disease. Implications for the mechanism of the training response. Circulation. 1994;90(4):1866–74.
223 . Multicenter randomized clinical trial of supervised exercise therapy with or without feedback versus walking advice for intermittent claudication. Journal of Vascular Surgery. 2010;52(2):348–55.
224 The Adjuvant Benefit of Angioplasty in Patients with Mild to Moderate Intermittent Claudication (MIMIC) Managed by Supervised Exercise, Smoking Cessation Advice and Best Medical Therapy: Results from Two Randomised Trials for Stenotic Femoropopliteal and Aortoiliac Arterial Disease. European Journal of Vascular and Endovascular Surgery. 2008;36(6):680–8.
225 . Walking performance and health-related quality of life after surgical or endovascular invasive versus non-invasive treatment for intermittent claudication – a prospective randomised trial. European Journal of Vascular and Endovascular Surgery. 2011;42(2):220–7.
226 . Is the generally accepted therapeutic benefit of exercise training in patients with peripheral arterial occlusive disease evidence based? Hamostaseologie. 2006;26(03):224–8.
227 Exercise Rehabilitation Improves Functional Outcomes and Peripheral Circulation in Patients with Intermittent Claudication: A Randomized Controlled Trial. Journal of the American Geriatrics Society. 2001;49(6):755–62.
228 . Exercise for intermittent claudication. The Cochrane Database of Systematic Reviews. 2017;12(12):CD000990.
229 Endovascular revascularization and supervised exercise for peripheral artery disease and intermittent claudication: A randomized clinical trial. JAMA. 2015;314(18):1936–44.
230 . A comparative study of upper body strength training exercise vs. treadmill walking on patients with intermittent claudication. Archives of Clinical Experimental Surgery. 2018;7(2):77–83.
231 . Exercise training for claudication. New England Journal of Medicine. 2002;347(24):1941–51.
232 . Calf muscle oxygen saturation and the effects of supervised exercise training for intermittent claudication. Journal of Vascular Surgery. 2012;56(2):470–5.
233 . Additional supervised exercise therapy after a percutaneous vascular intervention for peripheral arterial disease: a randomized clinical trial. Journal of Vascular and Interventional Radiology. 2011;22(7):961–8.
234 . Medical treatment of peripheral arterial disease and claudication. New England Journal of Medicine. 2001;344(21):1608–21.
235 . Cilostazol in the management of atherosclerosis. Current Vascular Pharmacology. 2010;8(3):363–72.
236 . Stansby GP. Cilostazol for peripheral arterial disease. Cochrane Database Syst Rev. 2008;23(1):CD003748.
237 Effect of cilostazol on treadmill walking, community-based walking ability, and health-related quality of life in patients with intermittent claudication due to peripheral arterial disease: meta-analysis of six randomized controlled trials. J Am Geriatr Soc. 2002;50(12):1939–46.
238 . Pentoxifylline for intermittent claudication. Cochrane Database Syst Rev. 2012;18(1):CD005262.
239 . Stansby G. Cilostazol for intermittent claudication. Cochrane Database Syst Rev. 2014;31(10):CD003748.
240 . The US experience with cilostazol in treating intermittent claudication. Atherosclerosis Supplements. 2005;6(4):21–31.
241 . The role of cilostazol (Pletal) in the management of intermittent claudication. International journal of clinical practice. 2003;57(5):405–9.
242 . Long-term safety of cilostazol in patients with peripheral artery disease: The CASTLE study (Cilostazol: A Study in Long-term Effects). Journal of Vascular Surgery. 2008;47(2):330–6.e2.
243 . Analysis of the cilostazol safety database. The American Journal of Cardiology. 2001;87(12, Supplement 1):28–33.
244 Cilostazol improves long-term patency after percutaneous transluminal angioplasty in hemodialysis patients with peripheral artery disease. Clin J Am Soc Nephrol. 2008;3(4):1034–40.
245 Cilostazol reduces target lesion revascularization after percutaneous transluminal angioplasty in the femoropopliteal artery. Circ J. 2005;69(10):1256–9.
246 Triple versus dual antiplatelet therapy after coronary stenting: impact on stent thrombosis. J Am Coll Cardiol. 2005;46(10):1833–7.
247 Cilostazol reduces angiographic restenosis after endovascular therapy for femoropopliteal lesions in the Sufficient Treatment of Peripheral Intervention by Cilostazol study. Circulation. 2013;127(23):2307–15.
248 Drug-eluting stenting followed by cilostazol treatment reduces late restenosis in patients with diabetes mellitus the DECLARE-DIABETES Trial (A Randomized Comparison of Triple Antiplatelet Therapy with Dual Antiplatelet Therapy After Drug-Eluting Stent Implantation in Diabetic Patients). J Am Coll Cardiol. 2008;51(12):1181–7.
249 Efficacy of cilostazol after endovascular therapy for femoropopliteal artery disease in patients with intermittent claudication. J Am Coll Cardiol. 2009;53(1):48–53.
250 . Pharmacologic approaches to restenosis prevention. Am J Cardiol. 2007;100(5A):10K–6K.
251 Referrals_document/Cilostazol_31/WC500140671.pdf. 2013.
252 . Naftidrofuryl for intermittent claudication. Cochrane Database Syst Rev. 2008;16(2):CD001368.
253 . Effect of naftidrofuryl on physiological walking distance in patients with intermittent claudication. Annales de cardiologie et d’angeiologie. 2001;50(3):175–82.
254 . Findings of the Naftidrofuryl in Quality of Life (NIQOL) European study program. Int Angiol. 2002;21(1):20–7.
255 Systematic review of the efficacy of cilostazol, naftidrofuryl oxalate and pentoxifylline for the treatment of intermittent claudication. Br J Surg. 2012;99(12):1630–8.
256 . Vander Stichele R. Buflomedil for intermittent claudication. Cochrane Database Syst Rev. 2013;28(3):CD000988.
257 . Ginkgo biloba for intermittent claudication. Cochrane Database Syst Rev. 2009;15(2):CD006888.
258 . Prostanoids for intermittent claudication. Cochrane Database Syst Rev. 2013;30(4):CD000986.
259 . A randomized trial of iloprost in patients with intermittent claudication. Vasc Med. 2008;13(1):5–13.
260 . The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719–24.
261 The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: Risk stratification based on Wound, Ischemia, and foot Infection (WIfI). Journal of Vascular Surgery. 2014;59(1):220–34.e2.
262 Science, medicine and the future: healing chronic wounds. BMJ. 2002;324(7330):160–3.
263 Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clinical Infectious Diseases. 2005;41(10):1373–406.
264 . Prostanoids for critical limb ischaemia. The Cochrane database of systematic reviews. 2018;1(1):CD006544-CD.
265 . Meta-analysis of randomised controlled prostaglandin E1 studies in peripheral arterial occlusive disease stages III and IV. Vasa. 2004;33(3):137–44.
266 . Treatment of patients with peripheral arterial occlusive disease fontaine stage IV with intravenous iloprost and PGE1: A randomized open controlled study. Prostaglandins, Leukotrienes and Essential Fatty Acids. 1993;49(2):573–8.
267 . A meta-analysis of randomized placebo control trials in Fontaine stages III and IV peripheral occlusive arterial disease. International Angiology: A journal of the International Union of Angiology. 1994;13(2):133–42.
268 . Prostanoids for critical limb ischaemia. Cochrane Database of Systematic Reviews. 2010(1).
269 Efficacy and Safety of Alprostadil in Patients with Peripheral Arterial Occlusive Disease Fontaine Stage IV: Results of a Placebo Controlled Randomised Multicentre Trial (ESPECIAL). European Journal of Vascular and Endovascular Surgery. 2017;53(4):559–66.
270 Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. The Lancet. 2002;360(9331):427–35.
271 Therapeutic angiogenesis with intramuscular NV1FGF improves amputation-free survival in patients with critical limb ischemia. Molecular Therapy. 2008;16(5):972–8.
272 Effect of fibroblast growth factor NV1FGF on amputation and death: a randomised placebo-controlled trial of gene therapy in critical limb ischaemia. The Lancet. 2011;377(9781):1929–37.
273 Effect of hypoxia-inducible factor-1alpha gene therapy on walking performance in patients with intermittent claudication. Circulation. 2011;124(16):1765–73.
274 A randomized, controlled study of autologous therapy with bone marrow – derived aldehyde dehydrogenase bright cells in patients with critical limb ischemia. Catheterization and Cardiovascular Interventions. 2011;78(7):1060–7.
275 . Safety and efficacy of autologous cell therapy in critical limb ischemia: a systematic review. 2013;22(3):545–62.
276 . Autologous bone marrow-derived cell therapy in patients with critical limb ischemia: a meta-analysis of randomized controlled clinical trials. Annals of Surgery. 2013;258(6):922–9.
277 Effect of repetitive intra-arterial infusion of bone marrow mononuclear cells in patients with no-option limb ischemia: the randomized, double-blind, placebo-controlled Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra-arterial Supplementation (JUVENTAS) trial. Circulation. 2015 Mar 10;131(10):851–60.
278 . Bone marrow derived cell therapy in critical limb ischemia: a meta-analysis of randomized placebo controlled trials. European Journal of Vascular and Endovascular Surgery. 2015;50(6):775–83.
279 Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. New England Journal of Medicine. 2003;348(7):593–600.
280 Bone marrow alterations and lower endothelial progenitor cell numbers in critical limb ischemia patients. PLoS One. 2013;8(1):e55592.
281 Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: A double-blind, randomized, controlled trial. Diabetes Research and Clinical Practice. 2011;92(1):26–36.
282 Characterization of mesenchymal stem cells of “no-options” patients with critical limb ischemia treated by autologous bone marrow mononuclear cells. PLoS One. 2013;8(9):e73722.
283 . Spinal cord stimulation for the treatment of vascular pathology. neurosurgery. Clinics. 2014;25(1):25–31.
284 . Spinal cord stimulation: a 20-year retrospective analysis in 260 patients. neuromodulation: technology at the neural. Interface. 2009;12(3):232–9.
285 . Spinal cord stimulation for non-reconstructable chronic critical leg ischaemia. Cochrane Database of Systematic Reviews. 2013(2).
286 . What is the evidence on efficacy of spinal cord stimulation in (subgroups of) patients with critical limb ischemia? Annals of Vascular Surgery. 2009;23(3):355–63.
287 . Oxygénothérapie hyperbare: Rapid Assessment. KCE. 2008;74B. https://kce.fgov.be/sites/default/files/atoms/files/d20081027314.pdf.
288 A systematic review and meta-analysis of adjunctive therapies in diabetic foot ulcers. Journal of Vascular Surgery. 2016;63(2, Supplement):46S–58S.e2.
289 Hyperbaric oxygen therapy does not reduce indications for amputation in patients with diabetes with nonhealing ulcers of the lower limb: a prospective, double-blind. Randomized Controlled Clinical Trial. Diabetes Care. 2016;39(3):392–9.
290 . Fibrinolytic agents for peripheral arterial occlusion. Cochrane Database Syst Rev. 2013;19(12):CD001099.
291 . A meta-analysis of the outcome of endovascular and noninvasive therapies in the treatment of intermittent claudication. Journal of Vascular Surgery. 2011;54(5):1511–21.
292 Peripheral Angioplasty as the First-choice Revascularization Procedure in Diabetic Patients with Critical Limb Ischemia: Prospective Study of 993 Consecutive Patients Hospitalized and Followed Between 1999 and 2003. European Journal of Vascular and Endovascular Surgery. 2005;29(6):620–7.
293 Outcome of infrainguinal revascularization for critical limb ischemia in diabetics with end stage renal disease. Vasa. 2006;35(1):15–20.
294 . Angiosome-targeted infrapopliteal endovascular revascularization for treatment of diabetic foot ulcers. Journal of Vascular Surgery. 2013;57(2):427–35.
295 . Diabetic foot and PAD: the endovascular approach. Diabetes/Metabolism Research and Reviews. 2012;28(S1):36–9.
296 . Covered endovascular reconstruction of aortic bifurcation (CERAB) technique: a new approach in treating extensive aortoiliac occlusive disease. J Cardiovasc Surg (Torino). 2013;54(3):383–7.
297 BRAVISSIMO: 12-month results from a large scale prospective trial. The Journal of Cardiovascular Surgery. 2013;54(2):235–53.
298 Outcomes of open operation for aortoiliac occlusive disease after failed endovascular therapy. Archives of Surgery. 2012;147(9):841–5.
299 Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: An intention-to-treat analysis of amputation-free and overall survival in patients randomized to a bypass surgery-first or a balloon angioplasty-first revascularization strategy. J Vasc Surg. 2010;51(5 Suppl):5S–17S.
300 Bypass versus angio plasty in severe ischaemia of the leg – 2 (BASIL-2) trial: study protocol for a randomised controlled trial. Trials. 2016;17(1):11.
301 . Stenting vs above knee polytetrafluoroethylene bypass for TransAtlantic Inter-Society Consensus-II C and D superficial femoral artery disease. Journal of Vascular Surgery. 2008;48(5):1166–74.
302 Multicentre randomised controlled trial of the clinical and cost-effectiveness of a bypass-surgery-first versus a balloon-angioplasty-first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial. Health technology assessment (Winchester, England). 2010;14(14):1–210, iii–iv.
303 . Superior limb salvage with endovascular therapy in octogenarians with critical limb ischemia. Journal of Vascular Surgery. 2009;50(2):305–16.e2.
304 . Four-year randomized prospective comparison of percutaneous ePTFE/nitinol self-expanding stent graft versus prosthetic femoral-popliteal bypass in the treatment of superficial femoral artery occlusive disease. Journal of Vascular Surgery. 2010;52(3):584–91.e7.
305 Nitinol stent implantation versus percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: the femoral artery stenting trial (FAST). Circulation. 2007;116(3):285–92.
306 Nitinol Stent Implantation vs. Balloon Angioplasty for Lesions in the Superficial Femoral and Proximal Popliteal Arteries of Patients With Claudication: Three-Year Follow-up From the RESILIENT Randomized Trial. Journal of Endovascular Therapy. 2012;19(1):1–9.
307 . Primary Nitinol Stenting in Femoropopliteal Occlusive Disease: A Meta-Analysis of Randomized Controlled Trials. Journal of Endovascular Therapy. 2012;19(5):585–95.
308 Balloon angioplasty versus stenting with nitinol stents in intermediate length superficial femoral artery lesions. Catheterization and Cardiovascular Interventions. 2009;74(7):1090–5.
309 Balloon Angioplasty versus Implantation of Nitinol Stents in the Superficial Femoral Artery. New England Journal of Medicine. 2006;354(18):1879–88.
310 Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation. 2007;115(21):2745–9.
311 Stent placement versus balloon angioplasty for the treatment of obstructive lesions of the popliteal artery: a prospective, multicenter, randomized trial. Circulation. 2013 Jun 25;127(25):2535–41.
312 MISAGO 2: One-year outcomes after implantation of the misago self-expanding nitinol stent in the superficial femoral and popliteal arteries of 744 patients. Journal of Endovascular Therapy. 2012;19(6):774–84.
313 Results of the Protégé EverFlex 200-mm-long nitinol stent (ev3) in TASC C and D femoropopliteal lesions. Journal of Vascular Surgery. 2011;54(4):1042–50.
314 Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circ Cardiovasc Interv. 2011;4(5):495–504.
315 Durable clinical effectiveness with paclitaxel-eluting stents in the femoropopliteal artery: 5-year results of the Zilver PTX randomized trial. Circulation. 2016;133(15):1472–83.
316 Durability of Treatment Effect Using a Drug-Coated Balloon for Femoropopliteal Lesions. 24-Month Results of INPACT SFA. J Am Coll Cardiol. 2015;66(21):2329–38.
317 Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. New England Journal of Medicine. 2015;373(2):145–53.
318 SUPERB final 3-year outcomes using interwoven nitinol biomimetic supera stent. Catheterization and Cardiovascular Interventions. 2017;89(7):1259–67.
319 Results from the Tack Optimized Balloon Angioplasty (TOBA) study demonstrate the benefits of minimal metal implants for dissection repair after angioplasty. Journal of Vascular Surgery. 2016;64(1):109–16.
320 . Risk of Death Following Application of Paclitaxel; Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta Analysis of Randomized Controlled Trials. Journal of the American Heart Association. 2018;7(24):e011245.
321 Safety of Paclitaxel-Coated Balloon Angioplasty for Femoropopliteal Peripheral Artery Disease. JACC: Cardiovascular Interventions. 2019.
322 Mortality after use of paclitaxel-based devices in peripheral arteries: a real-world safety analysis. European Heart Journal. 2019.
323 Infrapopliteal percutaneous transluminal angioplasty versus bypass surgery as first-line strategies in critical leg ischemia: a propensity score analysis. Annals of Surgery. 2010;252(5):765–73.
324 . The ongoing battle between infrapopliteal angioplasty and bypass surgery for critical limb ischemia. Annals of Vascular Surgery. 2012;26(8):1145–53.
325 Prospective trial of infrapopliteal artery balloon angioplasty for critical limb ischemia: angiographic and clinical results. Journal of Vascular and Interventional Radiology. 2000;11(8):1021–31.
326 . Balloon angioplasty in chronic critical limb ischemia: factors affecting clinical and angiographic outcome. Journal of Endovascular Therapy. 2002;9(4):403–10.
327 Lower limb multilevel treatment with drug-eluting balloons: 6-month results from the DEBELLUM randomized trial. Journal of Endovascular Therapy. 2012;19(5):571–80.
328 . Percutaneous transluminal angioplasty of infrapopliteal arteries in patients with intermittent claudication: Acute and one-year results. Catheterization and Cardiovascular Interventions. 2005;64(1):12–7.
329 Percutaneous transluminal angioplasty versus primary stenting in infrapopliteal arteries in critical limb ischemia. Vasa. 2011;40(6):482–90.
330 . Angioplasty or primary stenting for infrapopliteal lesions: results of a prospective randomized trial. CardioVascular and Interventional Radiology. 2010;33(2):260–9.
331 . Systematic review and meta-analysis of balloon angioplasty versus primary stenting in the infrapopliteal disease. Vascular and Endovascular Surgery. 2014;48(1):18–26.
332 . Preventing leg amputations in critical limb ischemia with below-the-knee drug-eluting stents. The PaRADISE (PReventing Amputations using Drug eluting StEnts) trial. J Am Coll Cardiol. 2010;55(15):1580–9.
333 Randomized comparison of everolimus-eluting versus bare-metal stents in patients with critical limb ischemia and infrapopliteal arterial occlusive disease. Journal of Vascular Surgery. 2012;55(2):390–8.
334 Meta-analysis of outcomes of endovascular treatment of infrapopliteal occlusive disease with drug-eluting stents. Journal of Endovascular Therapy. 2013;20(2):131–44.
335 Drug-eluting stents for revascularization of infrapopliteal arteries: updated meta-analysis of randomized trials. JACC: Cardiovascular Interventions. 2013;6(12):1284–93.
336 Sirolimus-eluting stents for treatment of infrapopliteal arteries reduce clinical event rate compared to bare-metal stents. Long-term results from a randomized trial. J Am Coll Cardiol. 2012;60(7):587–91.
337 A Prospective Randomized Multicenter Comparison of Balloon Angioplasty and Infrapopliteal Stenting With the Sirolimus-Eluting Stent in Patients With Ischemic Peripheral Arterial Disease. 1-Year Results From the ACHILLES Trial. J Am Coll Cardiol. 2012;60(22):2290–5.
338 Infrapopliteal application of sirolimus-eluting versus bare metal stents for critical limb ischemia: analysis of long-term angiographic and clinical outcome. Journal of Vascular and Interventional Radiology. 2009;20(9):1141–50.
339 Infragenicular Stent Implantation for Below-the-Knee Atherosclerotic Disease: Clinical Evidence from an International Collaborative Meta-Analysis on 640 Patients. Journal of Endovascular Therapy. 2009;16(3):251–63.
340 . Randomized Trials for Endovascular Treatment of Infrainguinal Arterial Disease: Systematic Review and Meta-analysis (Part 2: Below the Knee). European Journal of Vascular and Endovascular Surgery. 2014;47(5):536–44.
341 Percutaneous interventions below the knee in patients with critical limb ischemia using drug eluting stents. J Cardiovasc Surg (Torino). 2010 Apr;51(2):183–91.
342 . AMS INSIGHT – Absorbable Metal Stent Implantation for Treatment of Below-the-Knee Critical Limb Ischemia: 6-Month Analysis. CardioVascular and Interventional Radiology. 2009;32(3):424–35.
343 Everolimus-eluting bioresorbable vascular scaffolds versus everolimus-eluting metallic stents: a meta-analysis of randomised controlled trials. The Lancet. 2016;387(10018):537–44.
344 Drug-eluting balloon in peripheral intervention for below the knee angioplasty evaluation (DEBATE-BTK): a randomized trial in diabetic patients with critical limb ischemia. Circulation. 2013;128(6):615–21.
345 Drug-eluting balloon versus standard balloon angioplasty for infrapopliteal arterial revascularization in critical limb ischemia. 12-month results from the IN.PACT DEEP randomized trial. J Am Coll Cardiol. 2014;64(15):1568–76.
346 Paclitaxel-Coated Balloon in Infrapopliteal Arteries: 12-Month Results From the BIOLUX P-II Randomized Trial (BIOTRONIK’S-First in Man study of the Passeo-18 LUX drug releasing PTA Balloon Catheter vs. the uncoated Passeo-18 PTA balloon catheter in subjects requiring revascularization of infrapopliteal arteries). JACC: Cardiovascular Interventions. 2015;8(12):1614–22.
347 Single-center experience with lutonix drug-coated balloons in infrapopliteal arteries. Journal of Endovascular Therapy. 2016;23(3):417–23.
348 Drug-coated balloon angioplasty for the management of recurring infrapopliteal disease in diabetic patients with critical limb ischemia. Cardiovascular Revascularization Medicine. 2018;19(1, Part B):83–7.
349 Long-term results of direct and indirect endovascular revascularization based on the angiosome concept in patients with critical limb ischemia presenting with isolated below-the-knee lesions. Journal of Vascular Surgery. 2012;55(2):363–70.e5.
350 . Outcomes of angiosome and non-angiosome targeted revascularization in critical lower limb ischemia. Journal of Vascular Surgery. 2013;57(1):44–9.
351 A Reliable approach to diabetic neuroischemic foot wounds: below-the-knee angiosome-oriented angioplasty. Journal of Endovascular Therapy. 2011;18(3):376–87.
352 . Systematic review and meta-analysis of direct versus indirect angiosomal revascularisation of infrapopliteal arteries. European Journal of Vascular and Endovascular Surgery. 2014;48(1):88–97.
353 . The role of foot collateral vessels on ulcer healing and limb salvage after successful endovascular and surgical distal procedures according to an angiosome model. Vascular and Endovascular Surgery. 2010;44(8):654–60.
354 . Factors influencing wound healing of critical ischaemic foot after bypass surgery: is the angiosome important in selecting bypass target artery? European Journal of Vascular and Endovascular Surgery. 2012;43(3):322–8.
355 When is a technically successful peripheral angioplasty effective in preventing above-the-ankle amputation in diabetic patients with critical limb ischaemia? Diabetic Medicine. 2007;24(8):823–9.
356 PTA of infrapopliteal arteries: long-term clinical follow-up and analysis of factors influencing clinical outcome. CardioVascular and Interventional Radiology. 2010;33(4):720–5.
357 Percutaneous transluminal angioplasty for management of critical ischemia in arteries below the knee. Annals of Vascular Surgery. 2001;15(2):175–81.
358 . Debulking atherectomy in the peripheral arteries: is there a role and what is the evidence? CardioVascular and Interventional Radiology. 2017;40(7):964–77.
359 Endovascular therapy is effective treatment for focal stenoses in failing infrapopliteal vein grafts. Annals of Vascular Surgery. 2014;28(8):1823–31.
360 Meta-analysis of percutaneous transluminal atherectomy in the treatment for in-stent restenosis of lower extremity peripheral artery disease. 2015.
361 . Surgery versus thrombolysis for initial management of acute limb ischaemia. Cochrane Database Syst Rev. 2013;6(6):CD002784.
362 Clinical outcome of isolated popliteal artery aneurysms treated with a heparin-bonded stent graft. European Journal of Vascular and Endovascular Surgery. 2016;52(1):99–104.
363 . Endovascular repair of peripheral and visceral aneurysms with the cardiatis multilayer flow modulator: one-year results from the Italian Multicenter Registry. Journal of Endovascular Therapy. 2012;19(5):599–610.
364 Treatment of acute embolic occlusions of the subclavian and axillary arteries using a rotational thrombectomy device. Vasa. 2003;32(2):111–6.
365 Early experience with a rotational thrombectomy device for treatment of acute and subacute infra-aortic arterial occlusions. Journal of Endovascular Therapy. 2003;10(2):322–31.
366 Balloon angioplasty and stent implantation induce a vascular inflammatory reaction. Journal of Endovascular Therapy. 2002;9(1):59–66.
367 Inflammatory response to stent implantation: differences in femoropopliteal, iliac, and carotid arteries. Radiology. 2002;224(2):529–35.
368 . The three processes leading to post PTCA restenosis: dependence on the lesion substrate. Thromb Haemost. 1995 Jul;74(1):552–9.
369 . Predictors of Failure of Endovascular Revascularization for Critical Limb Ischemia. Scandinavian Journal of Surgery. 2012;101(3):170–6.
370 . Platelet activation is increased in peripheral arterial disease. Journal of Vascular Surgery. 2003;38(1):99–103.
371 . Reocclusion prophylaxis with dipyridamole combined with acetylsalicylic acid following PTA. Angiology. 1990;41(4):263–9.
372 Platelet inhibition with ASA/dipyridamole after percutaneous balloon angioplasty in patients with symptomatic lower limb arterial disease. A prospective double-blind trial. Study group on pharmacological treatment after PTA. Eur J Vasc Surg. 1994;8(1):83–8.
373 . Verhütung von Wiederverschlüssen nach Rekanalisation obliterierter Arterien mit der Kathetermethode. Deutsche medizinische Wochenschrift (1946). 1978;103(50):1994–7.
374 . Antiplatelet and anticoagulant drugs for prevention of restenosis/reocclusion following peripheral endovascular treatment. Cochrane Database of Systematic Reviews. 2005(1).
375 Controlled trial of high- versus low-dose aspirin treatment after percutaneous transluminal angioplasty in patients with peripheral vascular disease. Clin Investig. 1994;72(9):673–80.
376 Acetylsalicylic acid–reocclusion–prophylaxis after angioplasty (ARPA-study). A randomized double-blind trial of two different dosages of ASA in patients with peripheral occlusive arterial disease. VASA Zeitschrift fur Gefasskrankheiten. 1994;23(1):57–65.
377 Comparison of effects of high-dose and low-dose aspirin on restenosis after femoropopliteal percutaneous transluminal angioplasty. Circulation. 1995;91(8):2167–73.
378 Management of peripheral arterial interventions with mono or dual antiplatelet therapy – the MIRROR study: a randomised and double-blinded clinical trial. European Radiology. 2012;22(9):1998–2006.
379 A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. New England Journal of Medicine. 1998;339(23):1665–71.
380 2018 ESC/EACTS Guidelines on myocardial revascularization. European Heart Journal. 2018;40(2):87–165.
381 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134(10):e123–e55.
382 Cilostazol is associated with improved outcomes after peripheral endovascular interventions. Journal of Vascular Surgery. 2014;59(6):1607–14.
383 . Cilostazol and freedom from amputation after lower extremity revascularization. Journal of Vascular Surgery. 2015;61(4):960–4.
384 Clopidogrel with or without Omeprazole in Coronary Artery Disease. New England Journal of Medicine. 2010;363(20):1909–17.
385 Batroxobin for prevention of restenosis in diabetic patients after infrapopliteal arterial angioplasty: a small randomized pilot trial. Annals of Vascular Surgery. 2010;24(7):876–84.
386 Batroxobin plus aspirin reduces restenosis after angioplasty for arterial occlusive disease in diabetic patients with lower-limb ischemia. Journal of Vascular and Interventional Radiology. 2011;22(7):987–94.
387 . Low-molecular weight heparin versus aspirin and dipyridamole after femoropopliteal bypass grafting. The Lancet. 1994;344(8927):914–8.
388 . Low-molecular-weight heparin (reviparin) reduces the incidence of femoropopliteal in-stent stenosis: Preliminary results of an ongoing study. CardioVascular and Interventional Radiology. 1998;21(5):375–9.
389 . Potential use of a low-molecular-weight heparin to prevent restenosis in patients with extensive wall damage following peripheral angioplasty. Angiology. 2001;52(10):659–69.
390 Low-molecular-weight heparin for prevention of restenosis after femoropopliteal percutaneous transluminal angioplasty: A randomized controlled trial. Journal of Vascular Surgery. 2006;44(6):1247–53.
391 . Low-dose aspirin combined with dipyridamole versus anticoagulants after femoropopliteal percutaneous transluminal angioplasty. Radiology. 1994;193(2):567–71.
392 Nd:YAG laser with sapphire tip combined with balloon angioplasty in peripheral arterial occlusions. Long-term results. Circulation. 1991;83(1):141–7.
393 A review of antithrombotic therapy and the rationale and design of the randomized edoxaban in patients with peripheral artery disease (ePAD) trial adding edoxaban or clopidogrel to aspirin after femoropopliteal endovascular intervention. Journal of Endovascular Therapy. 2015;22(2):261–8.
394 . Pathophysiology of vein graft failure: A review. European Journal of Vascular and Endovascular Surgery. 1995;9(1):7–18.
395 . Effects of aspirin and dipyridamole on expanded polytetrafluoroethylene graft patency. Surgery. 1982;92(6):1016–26.
396 . A prospective randomized study to examine the effect of aspirin plus dipyridamole on the patency of prosthetic femoro-popliteal grafts. Vascular Surgery. 1984;18(4):217–21.
397 Effect of aspirin and dipyridamole on the patency of lower extremity bypass grafts. Surgery. 1984;96(3):462–6.
398 . Antiplatelet agents for preventing thrombosis after peripheral arterial bypass surgery. Cochrane Database of Systematic Reviews. 2015(2).
399 . A review of the role of anticoagulation in the treatment of peripheral arterial disease. Int J Angiol. 2012;21(4):187–94.
400 . Results of the randomized, placebo-controlled clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CASPAR) trial. Journal of Vascular Surgery. 2010;52(4):825–33.e2.
401 . Systematic review of randomized controlled trials of aspirin and oral anticoagulants in the prevention of graft occlusion and ischemic events after infrainguinal bypass surgery. Journal of Vascular Surgery. 1999;30(4):701–9.
402 Randomized controlled trial of dual antiplatelet therapy in patients undergoing surgery for critical limb ischemia. Ann Surg. 2010;252(1):37–42.
403 . Medikamentöse Rezidivprophylaxe nach femoropoplitealer Arterienrekonstruktion. Angio. 1979;2(1):73–7.
404 Efficacy of oral anticoagulants compared with aspirin after infrainguinal bypass surgery (The Dutch Bypass Oral anticoagulants or Aspirin study): a randomised trial. The Lancet. 2000;355(9201):346–51.
405 S2 Leitlinie zur Diagnostik und Therapie der Bein- und Beckenvenenthrombose und Lungenembolie. Vasa. 2010;39(Supplement 78):1–39.
406 Warfarin improves the outcome of infrainguinal vein bypass grafting at high risk for failure. Journal of Vascular Surgery. 1998;28(3):446–57.
407 . Benefits, morbidity, and mortality associated with long-term administration of oral anticoagulant therapy to patients with peripheral arterial bypass procedures: A prospective randomized study. Journal of Vascular Surgery. 2002;35(3):413–21.
408 Oral anticoagulant and antiplatelet therapy and peripheral arterial disease. New England Journal of Medicine. 2007;357(3):217–27.
409 . Combination therapy with warfarin plus clopidogrel improves outcomes in femoropopliteal bypass surgery patients. Journal of Vascular Surgery. 2012;56(1):96–105.
410 Impact of duration of statin medication on clinical outcomes in patients undergoing percutaneous transluminal angioplasty for atherosclerotic peripheral arterial disease. Journal of the American College of Cardiology. 2015;65(10 Supplement):A2045.
411 Perioperative management with antiplatelet and statin medication is associated with reduced mortality following vascular surgery. Journal of Vascular Surgery. 2014;59(6):1615–21.e1.
412 . Preoperative statins and limb salvage after lower extremity revascularization in the Medicare population. Circ Cardiovasc Interv. 2013;6(6):694–700.
413 Medication underuse during long-term follow-up in patients with peripheral arterial disease. Circ Cardiovasc Qual Outcomes. 2009;2(4):338–43.
414 . Age- and gender-related differences in the use of secondary medical prevention after primary vascular surgery: a nationwide follow-up study. European Journal of Vascular and Endovascular Surgery. 2012;43(3):300–7.
415 . Duplex scan surveillance during the first year after infrainguinal autologous vein bypass grafting surgery: Costs and clinical outcomes compared with other surveillance programs. Journal of Vascular Surgery. 2001;33(1):123–30.
416 Limb salvage after infrainguinal bypass graft failure. Journal of Vascular Surgery. 2004;39(5):951–7.
417 . Guidelines for critical limb ischaemia and diabetic foot – Introduction. European Journal of Vascular and Endovascular Surgery. 2011;42:S1–S3.
418 . Is duplex surveillance of value after leg vein bypass grafting?: Principal results of the Vein Graft Surveillance Randomised Trial (VGST). Circulation. 2005;112(13):1985–91.
419 . Effectiveness of a new exercise program after lower limb arterial blood flow surgery in patients with peripheral arterial disease: a randomized clinical trial. International Journal of Environmental Research and Public Health. 2014;11(8):7961.
420 . Sympathische Reflexdystrophie, Stumpf-und Phantomschmerz. Deutsches Ärzteblatt. 1994;91:1275–81.
421 . Prevalence and incidence of cardiovascular disease in 1160 older men and 2464 older women in a long-term health care facility. The Journals of Gerontology: Series A. 2002;57(1):M45–M6.
422 Prevalence of clinical and isolated subclinical cardiovascular disease in older adults with glucose disorders. The Cardiovascular Health Study. 2001;24(7):1233–9.
423 . The prevalence and one-year outcome of limb arterial obstructive disease in a nursing home population. Journal of the American Geriatrics Society. 1988;36(7):607–12.
424 . Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: the women’s health and aging study. Circulation. 2000;101(9):1007–12.
425 . Leg ulcers in peripheral arterial disease (arterial leg ulcers): Impaired wound healing above the threshold of chronic critical limb ischemia. Journal of the American Academy of Dermatology. 2000;43(6):1001–8.
426 . Prevalence of coexistence of coronary artery disease, ischemic stroke, and peripheral arterial disease in older persons, mean age 80 years, in an academic hospital-based geriatrics practice. Journal of the American Geriatrics Society. 1999;47(10):1255–6.
427 . Functional status of elderly adults before and after interventions for critical limb ischemia. Journal of Vascular Surgery. 2014;59(2):350–8.
428 . Geriatric syndromes in individuals admitted to vascular and urology surgical units. Journal of the American Geriatrics Society. 2014;62(6):1105–9.
429 . Treatment for critical lower limb ischemia in elderly patients. World Journal of Surgery. 2012;36(12):2937–43.
430 Factors related to postoperative delirium in patients with lower limb ischaemia: a prospective cohort study. European Journal of Vascular and Endovascular Surgery. 2012;44(4):411–5.
431 . Inpatient rehabilitation specifically designed for geriatric patients: systematic review and meta-analysis of randomised controlled trials. BMJ. 2010;20(340):c1718.