Novel cardiovascular biomarkers associated with peripheral arterial disease in men screened for abdominal aortic aneurysm
Abstract
Summary:Background: Peripheral arterial disease (PAD) is a common atherosclerotic disease with severity ranging from asymptomatic to chronic limb threatening ischemia. The aim of the present cross-sectional study was to identify novel biomarkers associated with PAD. Patients and methods: Levels of 91 cardiovascular specific proteins in plasma samples were measured by the Proseek Multiplex CVD III96x96 panel from a cohort consisting of 267 65-year-old men recruited from a screening program for abdominal aortic aneurysm (AAA) Levels of protein biomarkers were compared in men with and without PAD (defined as an ankle brachial index of <0.9) and their diagnostic potential was calculated by receiver-operating characteristic analysis. Results: The prevalence of PAD was 14.2% (38/267). After adjustment for multiple comparisons, levels of the following 11 biomarkers remained significantly higher (p<0.0001) in patients with PAD: secretoglobin family 3A member 2, osteoprotegerin, urokinase-type plasminogen activator surface receptor, serum macrophage chemokine ligand 16, matrix metalloproteinase 9, p-selectin, growth differentiation factor 15, elafin, cystatin B, trefoil factor 3, and fatty acid-binding protein 4. Multivariable logistic regression analysis (adjusted for smoking, use of antihypertensive and lipid-lowering medication, and metformin) showed that 11 biomarkers were significantly associated with higher risk of PAD with odds ratios ranging from 1.6 to 2.4. Area under curve calculated by receiver operating characteristic curve analysis (diagnostic value) for each protein biomarker ranged from 0.63 to 0.74. Conclusions: We have identified multiple proteins with a potential to be diagnostic biomarkers for PAD, and further research is warranted to clarify their potential predictive and prognostic value.
Introduction
Peripheral arterial disease (PAD) affecting lower extremity arteries commonly presents as intermittent claudication (IC), or chronic limb threatening ischemia (CLTI) [1]. A global report from 2015 estimated that 237 million people worldwide were affected by PAD, and that its prevalence increases with age [2]. The prevalence of PAD in Sweden in ages 60 to 90 years is 18% [3].
Hypertension, diabetes mellitus (DM), hypercholesterolaemia, smoking, and body-mass index (BMI) are well-established risk factors for PAD and other forms of atherosclerotic cardiovascular (CV) disease (CVD) [2]. Except for DM, these risk factors are also associated with development of abdominal aortic aneurysm (AAA) [4].
PAD and AAA patients are at high risk of CV mortality and morbidity, and consequently in need of secondary preventive measures with lipid-lowering, blood pressure management, smoking cessation, and in symptomatic cases also antiplatelet treatment [1, 5]. Early detection of both conditions is therefore of utmost importance.
PAD is commonly diagnosed as an ankle brachial index (ABI) <0.9 [3]. ABI measurement is, however, unreliable in patients with heavily calcified arteries, often present in patients with DM, renal insufficiency, and older age [6]. The pathophysiology of PAD features both inflammatory, atherosclerotic, and hypercoagulable mechanisms involving vascular endothelial cells, vascular smooth muscle cells, and inflammatory cells, suggesting that CV biomarkers might help identify PAD patients in need of prompt medical treatment to decrease morbidity and mortality [7]. Using proximity extension assay, we simultaneously screened 91 proteins associated with both various biological processes such as inflammation, angiogenesis, coagulation, platelet activation, cell adhesion, wound healing, chemotaxis, metabolism, and with AAA development for their potential role as new diagnostic biomarkers for PAD [8].
Patients and methods
Study population
In accordance with Swedish national recommendations, all 65-year-old men from the city of Malmö and 15 neighbouring municipalities are invited to ultrasound (US) screening for AAA at the Department of Vascular Diseases, Skåne University Hospital, Malmö [9, 10]. During 2010–2017, 415 (1.7%) of 24,589 examined men were diagnosed withAAA (aortic diameter ≥30 mm), out of whom 133 (32%) accepted toundergo physical examination, measurement of ABI, blood sampling, and assessment of medical history [11]. As acontrol group, we selected 134 men with aortic diameter <30 mm at screening, matched for comorbidities and date of examination and blood sampling. The whole study population was hereafter divided into groups consisting of patients with and without PAD, defined as an ABI <0.9 in one or both legs [3]. A further subdivision was based on the presence or absence of AAA.
Clinical variables
The following variables were analysed: systolic (S), and diastolic (D) blood pressure (BP), ABI in both legs, aortic diameter, plasma (p)-creatinine, p-cholesterol, p-triglycerides (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL) cholesterol, haemoglobin (Hb), p-glucose, p-homocysteine, smoking habits, cancer, cardiovascular disease, DM, use of antihypertensive, lipid-lowering drugs and metformin. Smoking habits were categorised into three groups: never, previous, and current smokers.
Blood sampling and biomarker quantification
As previously described in detail, we collected 6 ml fasting venous blood in vacuum tubes (Becton-Dickinson, Franklin Lakes, USA) containing ethylenediaminetetraacetic acid (EDTA) [8]. Samples were centrifuged at 1800 g for 15 min at 4C and plasma aliquoted and stored at 80°C for later analysis. Routine laboratory markers were analysed at the Department of Clinical Chemistry, Skåne University Hospital, Malmö (SWEDAC approved according to European norm 45001). Profiling of cardiovascular-related proteins was performed with Proseek Multiplex CVD III96x96 (Olink Biosciences) panel, a high-throughput, multiplex immunoassay allowing simultaneous measurement of 91 CVD-related proteins by Proximity Extension Assay (PEA) [12]. We performed PEA according to the Proseek Multiplex CVD III96x96 user manual at SciLifeLab at the Clinical Biomarker Facility, Uppsala University, Sweden [13]. In summary, 91 oligonucleotide-labelled antibody probe pairs that upon simultaneous binding to the target analyte creates a real-time PCR amplicon by proximity-dependent DNA polymerization. The resulting sequence is thereafter quantified using standard real-time PCR. Raw data were presented as Normalised Protein Expression (NPX) values by normalising cq-values against extension control, interplate control, and a correction factor. NPX values were on a log2 scale where a high NPX value corresponded with a high protein concentration and was linearized using the formula 2NPX. Limit of detection for each biomarker was determined based on the negative controls analysed in each run.
Statistical analysis
We used the Mann–Whitney U test for quantitative variables and the two-sided Fisher’s exact test for nominal variables, and the Bonferroni method for multiple-comparison correction. Nominal variables were stated as frequencies and percentages, and quantitative variables as median and interquartile range (IQR). We used univariate and multivariate logistic regression models to examine the association between biomarkers and clinical variables with PAD both in the whole material and in separate sub-analyses of men with and without AAA. Clinical variables differing significantly in univariate analysis (current smokers, diabetes mellitus, lipid-lowering and anti-hypertensive drug use) were included in a multivariable logistic regression model.
Receiver-operating characteristic (ROC) curves were analysed to evaluate the diagnostic potential of the different protein biomarkers for PAD. The plot represents the true positive rate (sensitivity) against the false positive rate (1-specificity), and the accuracy was measured as the area under the curve (AUC). An AUC value of 1 indicates a perfect test, whereas an AUC of 0.5 suggests that the test has no prognostic power. The Youden Index Method was used to establish the optimal cut-off point to discriminate the PAD patients from those without PAD. Statistical analyses were done in SPSS (SPSS Inc., IBM, New York, USA) version 20. Values of p<0.05 were considered as significant.
Ethical approval
All subjects gave written consent to participation and the study was approved by the Ethics Committee of Lund University (2010/239). All procedures were in accordance with the Declaration of Helsinki.
Results
Background characteristics
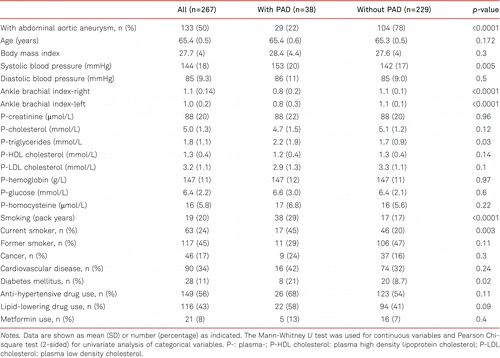
Baseline clinical characteristics of the 65-year-old men with and without PAD are shown in Table I. The overall prevalence of PAD was 14.2% (38/267); 21.8% (29/133) in patients with AAA and 6.7% (9/134) in patients without AAA. Patients in the PAD group had significantly higher SBP, TG, and prevalences of DM, lipid-lowering drug use, and smoking rates.
Biomarkers and PAD
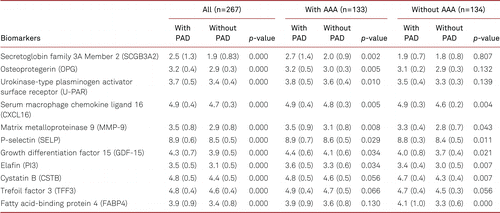
After correction for multiple comparisons, levels of the following 11 protein biomarkers were significantly higher (Table II) in men with PAD compared to subjects without PAD in the whole population (secretoglobin family 3A member 2 [SCGB3A2], osteoprotegerin [OPG], urokinase-type plasminogen activator surface receptor [U-PAR], serum macrophage chemokine ligand 16 [CXCL16], matrix metalloproteinase 9 [MMP-9], p-selectin [SELP], growth differentiation factor 15 [GDF-15], elafin [PI3], cystatin B [CSTB], trefoil factor 3 [TFF3], and fatty acid-binding protein 4 [FABP4]). To investigate the association of biomarkers with PAD in participants with and without AAA we performed subgroup analysis. Our results showed that all biomarkers except CSTB, TFF3, and FABP4 were significantly associated with higher risk of PAD in men with AAA, whereas in men without AAA all markers except SCGB3A2, OPG, U-PAR, and TFF3 were significantly associated with PAD. We also present biomarkers in patients with and without PAD subdivided on whether they had AAA or not (Electronic supplementary material [ESM] 1)
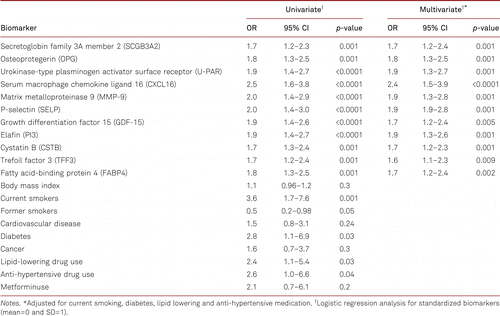
In univariate logistic regression analysis 11 potential biomarkers were significantly associated with higher risk of PAD (Table III). Among clinical variables included in the model, diabetes mellitus, lipid-lowering, and anti-hypertensive drug were significantly associated with the risk of PAD. Clinical variables significantly associated with PAD in univariate analysis were included in multivariate model showing that biomarkers were significantly associated with the risk of PAD independently of clinical variables included in the model (Table III). Among the biomarkers CXCL16 showed the strongest association with Odds Ratio (OR) of 2.5 (95% CI 1.3–2.4, p=0.001) in univariate and 2.4 (95% CI 1.2–2.3, p=0.001) in multivariate analysis. Odds ratios of all biomarkers are presented in Table III.
Diagnostic potential of biomarkers
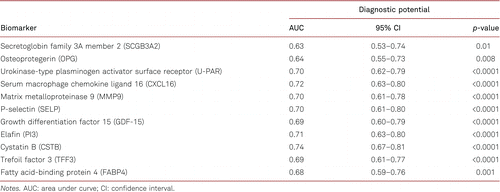
To evaluate the diagnostic value of the identified protein biomarkers, we performed ROC curve analysis. The AUC values and 95% CI of the protein biomarkers are shown in Table IV. Among all tested biomarkers, CSTB showed the best diagnostic potential for PAD (AUC 0.74, 95% CI 0.67–0.81, p<0.0001).
Discussion
The present study in 65-year-old men recruited from the general population demonstrated that increased plasma levels of 11 biomarkers; SCGB3A2, OPG, U-PAR, CXCL16, MMP-9, SELP, GDF-15, PI3, CSTB, TFF3, FABP4 were significantly associated with PAD. Furthermore, these biomarkers showed a significant diagnostic potential for PAD. To our knowledge, the present study is the first to show a significant association between SCGB3A2, U-PAR, SELP, PI3, CSTB, TFF3, and atherosclerotic PAD in the lower extremities.
SCGB3A2 is a small molecular weight secreted protein in airway epithelial cells involved in lung development, inflammatory reactions, and asthma [14]. Plasma SCGB3A2 has been identified as a promising biomarker for chronic heart failure [15].
The urokinase-type plasminogen activator and its specific receptor (U-PAR) are involved in vascular remodelling by controlling vascular smooth muscle cell migration and proliferation [16]. Furthermore, soluble urokinase-type plasminogen activator receptor derived from (U-PAR) is associated with inflammation and thrombogenesis, prevalent PAD, as well as with both all cause- and CV mortality [17].
SELP has not previously been related to PAD. Elevated levels of P-selectin, a glycoprotein encoded by SELP localized in the granules of platelets and Weibel-Palade bodies of endothelial cells, were associated with lower ABI and prevalent PAD in a prospective population-based cohort of 6,814 CV healthy individuals [18]. Furthermore, P-selectin also predicted incident PAD and progression from a normal to pathological ABI. P-selectin did, however, not appear to add incremental predictive ability beyond traditional risk factors, or traditional risk factors plus markers of inflammation and coagulation [19].
Increasing levels of the endogenous protein PI3 inhibiting neutrophil-mediated inflammation protect the CV system from a range of diseases [20]. Elevated levels of PI3 in patients with PAD might therefore be a result of a physiological response to the neutrophil-mediated inflammatory processes leading to atherosclerosis.
CSTB is widely expressed in most human cells and has been proposed as a biomarker for cancer and CV disease [21]. Results from Malmö Diet and Cancer study showed a significant association between CSTB levels and incident coronary events [21]. Additionally, CSTB levels are correlated with metabolic syndrome and increased in individuals with DM [21]. Such associations might perhaps also be relevant in PAD.
TFF3 is mainly secreted from goblet cells in the gastrointestinal system and has been associated with inflammation and malignancies [22]. TFF3 has shown to have cardioprotective effects by reducing infarct size when administered immediately after myocardial ischemia in mice [22]. Increased TFF3 levels in PAD might reflect compensatory vasoprotective effects.
Furthermore, our results corroborate previous associations of OPG, CXCL16, MMP-9, GDF-15, and FABP4 with non-coronary atherosclerotic disease [23, 24, 25, 26, 27].
OPG is a protein of the tumour necrosis factor receptor family and part of the RANK/RANKL/OPG system, playing active parts in atherogenesis and arterial calcification [23]. In a comparably smaller study, serum OPG was measured in 165 patients with type 2 DM, of which 119 patients had PAD based on toe-brachial index [23]. Significant associations were shown between serum OPG levels and both presence and severity of PAD.
No previous relationships have been reported between CXCL16, a transmembrane chemokine expressed on endothelial cells and platelets and PAD in the lower extremities. Among patients with acute ischemic stroke, however, increased CXCL16 levels were related to carotid artery intima-media thickness, plaque area, lumen stenosis rate, and increased plaque vulnerability [24].
MMPs facilitate extracellular matrix turnover in arterial remodelling in response to occluded or stenotic arterial segments [25]. In a cross-sectional cohort of 121 patients, higher levels of MMP-9 were reported in patients with PAD. CLTI patients showed the highest levels of MMP-9, and the authors speculate that ischemic muscle might be a potential source of MMP-9 [25].
GDF15 is a member of the transforming growth factor family and a promising prognostic biomarker for CV disease [26]. In two cohorts with 546 patients, elevated levels of GDF15 in PAD patients were associated with increased total mortality and rate of major amputation during 3 years [26]. Furthermore, patients with GDF15 levels in the highest tertile had more than three times higher risk of major amputation or death compared to those with levels in the lowest tertile, and levels of GDF15 were highly correlated with disease severity [26].
FABP4, also known as adipocyte FABP, is an intracellular lipid chaperon produced and released by adipocytes [27]. In a cohort of 327 PAD patients, serum FABP4 levels showed a significant association with a composite of death, nonfatal myocardial infarction, or nonfatal stroke during five years, and were associated with higher all-cause mortality [27].
Among the studied biomarkers, significant positive correlations were previously reported between U-PAR, SELP, GDF15, PI3, CSTB, and FABP4 and aortic diameter among patients with AAA in our material [8]. In another study in which 329 AAA were biopsied during surgery, OPG concentrations were also positively associated with AAA diameter, and markers of proteolysis and inflammation [28].
The different biomarker patterns in PAD patients with and without AAA the present study should be evaluated in this context. The fact that SCGB3A2, OPG, U-PAR, and TFF3 were not associated with PAD in the group without AAA, whereas CSTB, TFF3, and FABP4 were not associated with PAD in the group with AAA might either be due to the smaller sizes of the subgroups, or to the fact that an AAA might in some way modulate the relationships between PAD and biomarkers.
European guidelines recommend medical treatment for symptomatic PAD and for all AAA patients including smoking cessation, blood pressure management, anti-platelet and lipid-lowering medication to improve limb symptoms, reduce CV events, and mortality [1, 29]. There is currently no effective medication to reduce AAA growth rate, however [29]. Identification of individuals at risk of PAD by using biomarkers such as SCGB3A2, OPG, U-PAR, CXCL16, MMP-9, SELP, GDF-15, PI3, CSTB, TFF3, or FABP4 may lead to earlier identification of individuals at risk. Earlier initiation of appropriate medical treatment may counteract CV risk factors at an early stage. The diagnostic potential of the studied biomarkers could therefore make them relevant in future PAD screening, and further research with a longitudinal prospective regarding their prognostic potential could strengthen their role in clinical use.
Limitations
Our study has inherent limitations; due to its cross-sectional design we cannot conclude whether the associations represent causes or effects. Furthermore, some risk for confounding always remains in non-randomized studies despite multivariate adjustment attempts. Since all study subjects were men of similar age, we are also unable to account for any potential gender- or age specific changes related to biomarker levels. As in all cohort studies, the potential risk of selection bias when including participants cannot be excluded. The main strength of the current study is the relatively large study population, and reliable measurements of ABI and aortic diameter.
Conclusions
The studied biomarkers may potentially be used to identify PAD in men, irrespectively of the presence of AAA or not. However, further research is needed to evaluate their potential predictive and prognostic value.
Electronic supplementary material
The electronic supplementary material (ESM) is available with the online version of the article at https://doi.org/10.1024/0301-1526/a000999
References
1 Editor’s Choice - 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2018;55(3):305–68.
2 Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. 2019;7(8):e1020–30.
3 A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J Vasc Surg. 2007;45(6):1185–91.
4 Abdominal aortic aneurysms. Nat Rev Dis Primers. 2018;4(1):34.
5 . Medical management of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2007;34(3):267–73.
6 . Peripheral arterial disease: diagnostic challenges and how photoplethysmography may help. Br J Gen Pract. 2015;65(635):323–4.
7 . A review of the pathophysiology and potential biomarkers for peripheral artery disease. Int J Mol Sci. 2015;16(5):11294–322.
8 Associations of global DNA methylation and homocysteine levels with abdominal aortic aneurysm: A cohort study from a population-based screening program in Sweden. Int J Cardiol. 2020;321:137–42.
9 Outcome of the Swedish Nationwide Abdominal Aortic Aneurysm Screening Program. Circulation. 2016;134(16):1141–8.
10 The importance of socioeconomic factors for compliance and outcome at screening for abdominal aortic aneurysm in 65-year-old men. J Vasc Surg. 2013;58(1):50–5.
11 . Inflammatory markers associated with abdominal aortic aneurysm. Eur Cytokine Netw. 2016;27(3):75–80.
12 Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014;9(4):e95192.
13 . Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res. 2011;39(15):e102.
14 Association between secretoglobin family 3A member 2 (SCGB3A2) gene polymorphisms and asthma in a Korean Population. Med Sci Monit. 2017;23:1880–5.
15 Utility of temporal profiles of new cardio-renal and pulmonary candidate biomarkers in chronic heart failure. Int J Cardiol. 2019;276:157–65.
16 Urokinase receptor associates with myocardin to control vascular smooth muscle cells phenotype in vascular disease. Arterioscler Thromb Vasc Biol. 2012;32(1):110–22.
17 Circulating soluble urokinase plasminogen activator receptor levels and peripheral arterial disease outcomes. Atherosclerosis. 2017;264:108–14.
18 . P-selectin in arterial thrombosis. Z Kardiol. 2004;93(11):855–63.
19 Soluble P-selectin predicts lower extremity peripheral artery disease incidence and change in the ankle brachial index: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2015;239(2):405–11.
20 . Role of the endogenous elastase inhibitor, elafin, in cardiovascular injury: from epithelium to endothelium. Biochem Pharmacol. 2012;83(6):695–704.
21 High levels of cathepsin D and cystatin B are associated with increased risk of coronary events. Open Heart. 2016;3(1):e000353.
22 Cardioprotective proteins upregulated in the liver in response to experimental myocardial ischemia. Am J Physiol Heart Circ Physiol. 2012;303(12):H1446–58.
23 . Osteoprotegerin concentration is associated with the presence and severity of peripheral arterial disease in type 2 diabetes mellitus. VASA. 2018;47(2):131–5.
24 . The relationship between serum CXCL16 level and carotid vulnerable plaque in patients with ischemic stroke. Eur Rev Med Pharmacol Sci. 2017;21(17):3911–5.
25 . Abnormal circulating levels of metalloprotease 9 and its tissue inhibitor 1 in angiographically proven peripheral arterial disease: relationship to disease severity. J Intern Med. 2005;257(1):110–6.
26 Growth differentiation factor 15 is associated with major amputation and mortality in patients with peripheral artery disease. J Am Heart Assoc. 2017;6(9):e006225.
27 . FABP4 and cardiovascular events in peripheral arterial disease. Angiology. 2018;69:424–30.
28 Osteoprotegerin is associated with aneurysm diameter and proteolysis in abdominal aortic aneurysm disease. Arterioscler Thromb Vasc Biol. 2012;32(6):1497–504.
29 Editor’s Choice - European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur J Vasc Endovasc Surg. 2019;57(1):8–93.



