Promoting urinary continence with nonpharmacological interventions in Parkinson’s disease
A nonrandomized controlled trial
Abstract
Abstract.Background: The evidence of nonpharmacological interventions promoting urinary continence in Parkinson’s disease is low and rehabilitation nurses do not give it a high priority. Aim: To examine the effects of a urinary incontinence management program on continence and quality of life in persons with Parkinson’s disease in inpatient rehabilitation. The acceptance of the intervention and the knowledge of the nurses were also of interest. Methods: A nonrandomized experimental study was conducted. The data collection commenced with a control group followed by an intervention group. A structured urinary incontinence management was performed in the intervention phase. This includes incontinence assessment, care planning, individual interventions, discharge planning, and telephone support at home. Results: Urinary continence and quality of life were enhanced in both groups; however, improvements were higher in the intervention group but the differences were not significant. Knowledge of the nurses about urinary incontinence improved during the study duration. The acceptance of the intervention was high. Conclusions: A structured urinary continence management may improve urinary continence in persons suffering from Parkinson’s disease. In clinical practice it is important to perform a detailed assessment to identify problems regarding urinary incontinence.
Zusammenfassung.Hintergrund: Die Evidenz für nicht-pharmakologische Interventionen zur Förderung der Harnkontinenz bei der Parkinson-Krankheit ist gering und hat bei Rehabilitationspflegenden keine hohe Priorität. Ziel: Es wurden die Auswirkungen eines Programms zur Behandlung der Harninkontinenz auf die Kontinenz und die Lebensqualität von Menschen mit der Parkinson-Krankheit während dem stationären Rehabilitationsaufenthalt untersucht. Von Interesse waren auch die Akzeptanz der Intervention und das Wissen des Pflegepersonals. Methoden: Es wurde eine nicht-randomisierte experimentelle Studie durchgeführt. Die Datenerhebung begann mit einer Kontrollgruppe, gefolgt von einer Interventionsgruppe. In der Interventionsphase wurde eine strukturierte Harninkontinenzversorgung durchgeführt. Diese umfasst Inkontinenzbeurteilung, Pflegeplanung, individuelle Interventionen, Entlassungsplanung und telefonische Unterstützung zu Hause. Ergebnisse: Harninkontinenz und Lebensqualität verbesserten sich in beiden Gruppen; in der Interventionsgruppe waren die Unterschiede jedoch höher, waren jedoch nicht signifikant. Das Wissen des Pflegepersonals über Harninkontinenz steigerte sich während der Studiendauer. Die Akzeptanz der Intervention war hoch. Schlussfolgerungen: Eine strukturierte Harninkontinenzversorgung kann die Harninkontinenz bei Parkinson-Patient_innen verbessern. In der klinischen Praxis ist es wichtig, eine detaillierte Beurteilung durchzuführen, um Probleme der Harninkontinenz zu identifizieren.
What is already known?
It is important to perform a detailed assessment to identify problems regarding urinary incontinence.
What is new?
Promoting urinary continence in persons suffering from Parkinson’s disease can positively impact their quality of life.
What are the practical implications?
Patients are open to interventions regarding promoting urinary continence and are motivated to improve their situation with non-pharmacological interventions.
Introduction
Parkinson’s disease (PD) is, after Alzheimer’s, the second most common neurodegenerative disorder with an incidence rate of 160 per 100,000 people aged 65 years or older in high-income countries (Ascherio & Schwarzschild, 2016; Hirtz et al., 2007). The incidence of PD is low before the age of 50 years and peaks at around 80 years (Ascherio & Schwarzschild, 2016). Exposure to pesticides, consumption of dairy products, history of melanoma, and traumatic brain injury has been associated with increased risk of PD. Currently, the promotion of physical activity is likely to be the only beneficial intervention to prevent PD (Ascherio & Schwarzschild, 2016). The diagnosis of PD is based on the presence of bradykinesia and either resting tremor or rigidity, with the current state of knowledge, Levodopa remains the mainstay of treatment for PD (Reich & Savitt, 2019). Although PD is considered a purely motor disorder, there is an exhaustive list of non-motor manifestations equally, if not more, disabling and significantly affecting the quality of life (Reich & Savitt, 2019). Lower urinary tract symptoms as a non-motor symptom of PD are very common and effecting; the prevalence is between 27 and 85 % (McDonald, Winge & Burn, 2017). Regarding to Winge (2015), about 30 % suffer from urinary incontinence. Excluded from this prevalence is the functional incontinence which would increase this number significantly. A prevalence survey in a rehabilitation clinic in Switzerland has revealed that 50.3 % of people with PD suffer from urinary incontinence at onset (Meixner, Saxer & Kohler, 2018). In a qualitative study, Burgstaller and Saxer (2017) summarized that urinary incontinence in persons suffering from PD is an emotional and social problem and it lacks adequate professional support of nurses and physicians. Tapia et al. (2013) concluded in their review that the health related quality of life is lower in the case of urinary incontinence if the person also suffers from a neurological disease. Symptoms associated with an overactive bladder are common. Bladder training and anticholinergic medication may have a role, however, the evidence is low (McDonald et al., 2017). Regarding to the UK National Health Service (2019) conservative treatments, which do not involve medicines or surgery, should be attempted first. These include, for example, lifestyle changes, pelvic floor muscle training, or bladder training.
There is low evidence of effective intervention for the nonpharmacological treatment of urinary incontinence in PD. In a systematic review, Hajebrahimi, Chapple, Pashazadeh and Salehi-Pourmehr (2019) included 41 studies with a total of 1063 patients that evaluated pharmacological, neurosurgical, botulin toxin, electrical neuromodulation, and behavioral therapy effects on a neurogenic bladder in patients with PD. The authors concluded that at present there is little to no evidence that treatment improves outcomes. They could only find two pilot studies investigating conservative non-pharmacological interventions (pelvic floor muscle exercises and bladder control strategies), one study with a high risk of bias, the other unclear (Hajebrahimi et al., 2019). Only one study could be found, which was published after the review mentioned before. This study examined the efficacy of behavioral therapy compared to control among people with Parkinson’s disease. The intervention consisted of pelvic floor muscle exercises and bladder training, as well as fluid and constipation management. There were no significant differences found regarding mean weekly incontinence episodes or overactive bladder symptoms but the intervention group reported significant higher quality of life and less inconvenience after the behavioral therapy (Vaughan et al., 2019).
Although evidence of nonpharmacological interventions promoting urinary continence in PD is low, in the general population, some evidence of behavioral interventions promoting continence can be found (Eustice et al., 2009; Imamura et al., 2015; Ostaszkiewicz et al., 2010a; Ostaszkiewicz et al., 2010b; Wallace et al., 2004). Practically, it makes sense that the rehabilitation nurse leads in promoting continence, but there is some lack of knowledge. A study about promoting continence in stroke rehabilitation concluded that nurses reactively manage urinary incontinence, adopting a routinized approach based on local custom and practice and promotion of urinary continence is not a priority in stroke rehabilitation for nurses (Booth, Kumlien, Zang, Gustafsson & Tolson, 2009).
Aim and research questions
In summary, it can be stated that the evidence of nonpharmacological interventions promoting urinary continence in PD is low and rehabilitation nurses do not give it a high priority. But since there is evidence in general, further research is justified. This is congruent with the statement of Cave (2017), who concluded that ongoing research is needed regarding continence interventions for acute rehabilitation patients. Therefore, the aim of the current study was a long-term improvement in continence and thus in the quality of life of patients with PD. The research questions were: Does structured, nurse-led urinary incontinence management improve urinary continence and the quality of life of patients with PD compared to conventional care during stationary rehabilitation? Do training courses on “Urinary incontinence in people with Parkinson’s disease” improve nurses’ knowledge about urinary incontinence? And what is the experience of the participants with PD in the treatment of urinary incontinence?
Methods
Design
This was a nonrandomized experimental study. The data collection started with a control group followed by an intervention group. Participants could not be randomized because at first all were included in the control group, and then all were included in the intervention group. It was impossible to maintain both groups at the same time because after the intervention was implemented, the knowledge and skills of the nurses would have influenced their maintenance of the control group. This procedure (two consecutive phases, on the same ward with the same staff) also has advantages. It is guaranteed that intervention and control can be carried out under the same structural and personnel conditions. Using another institution as a control group would be a possibility, but there is no comparable rehabilitation clinic in German-speaking Switzerland, the other rehabilitation clinics have different structures, e. g. no specialist ward for Parkinson’s disease or different distribution of roles in the interprofessional team.
The study is registered in the German Clinical Trials Register Nr. DRKS00007973. For the selection of the reporting guidelines, the equator network recommendation was followed and the TREND statement checklist (Des Jarlais, Lyles & Crepaz, 2004) was chosen for nonrandomized evaluations of behavioral and public health interventions as well as the TIDieR Checklist (Hoffmann et al., 2014).
Additionally, the last five participants of the intervention group were included in a qualitative sub-study to investigate their experience of the newly implemented systematic incontinence management with the focus on acceptance and examination of one’s own situation.
Setting and participants
The study was conducted at a Parkinson’s Center in the German-speaking part of Switzerland with a capacity of 23 beds. The planned convenience sample was 50 participants (30 participants in the control group and 20 in the intervention group). Due to the lack of reference values from comparable studies, it was not possible to conduct an adequate power analysis. Persons were included if they suffered from idiopathic Parkinson’s disease, regardless of the subtype (akinetic rigid type, tremor dominant type or monosymptomatic rest tremor type), if they suffered from urinary incontinence (as defined by the International Continence Society), if they would live at home or in a nursing home again after the rehabilitation stay, if they were able to communicate in the German language, and if they gave their written consent to participate in the study. Persons with a suprapubic or transurethral permanent catheter, with a Mini-Mental Status Test value of less than 18, with a medically diagnosed depression, or with an inability to complete a questionnaire due to e. g. delirium or acute hallucinations, as well as those who would not live at home after the rehabilitation stay (but in an institution, e. g. in a nursing home – “change in setting”), were excluded from the study.
Patients were informed about the study in advance by a letter a few weeks before entering the clinic. On the day of admission, they were screened by the ward nurse or the Parkinson’s nurse, and if they were suitable, the nurse asked the patient to participate in the study – the decision was made by the patient on the following day.
Control and intervention
During the control phase, patients received the usual care. On admission to the rehabilitation clinic, a micturition protocol was kept for 24 hours and from this, measures for dealing with incontinence were partly derived and carried out unsystematically for example toilet training. The evaluation of the measures was carried out in an unstructured and often random manner. There was no specific exit interview to promote urinary incontinence at home, and there was no support at home by the nursing staff after leaving the rehabilitation clinic.
A structured urinary incontinence management was performed by the registered nurses in the intervention phase. This includes incontinence assessment, care planning, individual interventions, discharge planning, and telephone support at home (see Table 1), this means that the intervention continuous the whole inpatient rehabilitation stay. This intervention consisted of several dependent and independent interacting components and thus represented a complex intervention. The intervention was developed by the project team, based on the findings of literature and extensive experience. The project team includes two people who have in-depth knowledge in the field of continence promotion and who also wrote a dissertation in this field (nursing science), as well as people who have a lot of practical experience in neurological rehabilitation as nurses who worked on the development of the intervention. In addition, a neurologist with in-depth knowledge in the medical treatment of Parkinson’s disease was involved in the project.

The selection of the individual measures was guided by an algorithm, which was led by the results of the assessments. That means that on account of the assessment, the nurses were guided to the right interventions, for example, whether an individual adopted toilet training or bladder training. To implement the intervention, all registered nurses of the ward were trained in one and a half days. On the first day, the basics of promoting urinary continence were repeated, and after this, all the steps of the intervention were discussed in detail. On the second half-day, two cases were analyzed in groups and discussed afterward regarding the intervention. Nonregistered nurses were trained about part of the intervention, especially the practical implementation of the measures during the rehabilitation stay, during a half-day. The training was guided by two nurses of the project team, which has extended knowledge in promoting continence as well as in rehabilitation nursing. The intervention was documented in detail in a booklet. This means that the assessment, the interventions which were based on it as well as the exit planning were recorded. This ensured that the intervention was carried out correctly for all patients and adverse events were documented.
Ethical considerations
The study was approved by the responsible ethics committee (KEKTGOV2015 / 03). Prior written informed consent was obtained by the ward nurse after the participants verbally agreed to participate in the study. They were informed that they could withdraw from the study at any time without stating a reason.
Data collection
Data were collected from July 2015 until September 2016 (control phase) and from January 2017 until April 2019 (intervention phase). The ward manager or the Parkinson’s nurse handed out the questionnaires to the participants, if necessary, e. g. if they could not write, they helped them to fill them in. For the measurements after discharge, the participants were given the corresponding questionnaires with an answer envelope upon discharge. The nurses and the data collectors were not blinded. The written and verbal patient information was designed in such a way that the participants were not told which group they were in. It must be noted, however, that the participants in the intervention group in particular were very likely to recognise the group to which they belonged, as a great deal of effort was put into the assessment. And the last five participants were interviewed for the intervention; at least from the time of the interview request, they were no longer blinded. The primary outcomes, continence and quality of life, were assessed at entry (t01), discharge (t02), one month after discharge (t03), and three months after discharge (t04). The knowledge of the nurses was measured at the beginning of the study, before the intervention was implemented, and at the end of the study.
To measure the extent of incontinence and incontinence-induced distress, the ICIQ-UI-SF questionnaire (International Consultation on Incontinence Modular Questionnaire – Short Form) was employed. The three scored items of the ICIQ are the frequency of involuntary urine leakage, the amount of involuntary urine lost and the impairment in daily life due to incontinence. These three answers result in a sum, with minimum score of 0, and maximum score of 21. Klovning et al. (2009) proposed the following severity intervals for the ICIQ slight (1 – 5), moderate (6 – 12), severe (13 – 18), and very severe (19 – 21). The ICIQ also comprises a fourth non-scored self-diagnostic item included by the expert committee because it was thought to be useful in clinical practice, to understand patients’ perception of the cause and type of leakage. Additionally, a fifth question was added in this study to measure the use of incontinence pads. The ICIQ-SF questionnaire is recommended by the International Consultation on Incontinence Symptoms and Quality of Life Committee and has now been translated into 50 languages. Psychometric testing of the instrument has already been carried out in many countries, and it has been demonstrated to be reliable and valid throughout (Avery et al., 2004). To assess the incontinence-associated quality of life, the I-QOL was chosen. The “Incontinence Quality of Life Instrument” contains 22 items on a 5-point Likert scale and focuses on the patient’s perspective. A total score of 110 points is possible; subscales are also formed (avoidance behaviors, psychosocial impacts, and social embarrassment). The higher the score, the better the quality of life (Martin & Brazg, 2010). The instrument was developed in English and translated into French, Spanish, Swedish, and German. It has high reliability: The Cronbach’s Alpha coefficient in all languages is between 0.87 and 0.93 for the overall score (Patrick et al., 1999). The knowledge of the nurses about incontinence was assessed by a validated questionnaire of Saxer, Bie, Dassen and Halfens (2008), which assesses the knowledge of nurses about incontinence and care of incontinent residents in nursing homes. The questionnaire consists of 18 items related to urinary incontinence, risk factors, and treatment options. The answer options are “Yes,” “No,” and “Don’t know.” In order to specifically investigate the knowledge about incontinence in patients with PD, the questionnaire was further developed and has been tested in practice for comprehensibility before the study started. Six of the existing questions were adapted and three new questions were added to fit the setting and address specific problem areas. The questionnaire was submitted to seven German-speaking nursing experts in the field of rehabilitation and / or incontinence for validation and each question was assessed using the following scale: Not relevant; Not assessable, Needs little adaptation (please insert suggestion); Very relevant. Overall, the questions were always rated as very relevant by a large majority. Based on the feedback, minor adjustments were made to the content of five questions. This was done to optimise the comprehensibility of the question or to make it more precise. This means that the instrument used in the current study consists of 21 items after validation and can be evaluated by adding up the number of correctly answered questions.
Data analysis
The participants’ characteristics were analyzed with the standard measures of means, standard deviations, and frequencies. The study variables were also analyzed with standard measures before and after the intervention for each group separately. Differences in the study variables before and after the intervention were assessed within the groups by a nonparametric test for related samples, the Friedman test. A two-tailed alpha of 0.05 described statistical significance. Statistical analyses were performed using SPSS, version 24 with an intention to treat approach.
Collection of participants’ experiences about the intervention
The last five participants of the intervention group additionally took part in a qualitative study. They were interviewed during the last week of their stay in inpatient rehabilitation. The aim was to record how incontinent people with PD experience the newly implemented systematic incontinence management with a focus on the acceptance and examination of one’s own situation. The interviews were conducted by the research team (first author) and were audiotaped and transcribed verbatim. It was performed by a qualitative structuring content analysis with an inductive approach, which is close to Mayring (Mayring, 2015).
Results
Participants characteristics
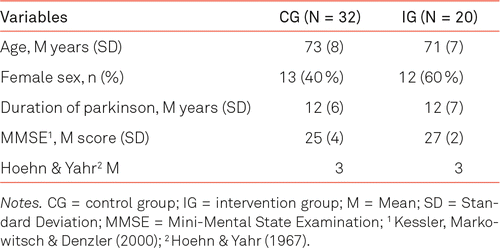
A total of 52 participants could be included, 25 of whom were women. There were 32 patients in the control group and 20 in the intervention group. Further details are given in Table 2. All differences in participants’ characteristics at baseline between the control and intervention group were not significant.
Patients outcome
In the intervention, it was possible that several measures to promote urinary continence were initiated simultaneously at least one measure was carried out for each participant. Adaptation of drinking behavior was the most frequently chosen intervention. It was conducted in 14 patients of the intervention group. Individual toilet training was carried out in 13 cases and, in one case, bladder training was initialized. By six people, the incontinence material was adjusted, and also by six people special measures were planned for the night, for example, a urinal. No adverse events were reported.
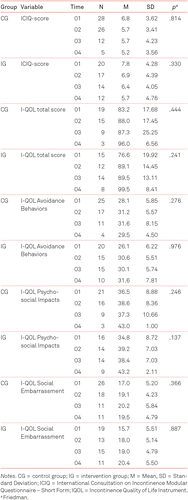
In the control and the intervention group, incontinence symptoms were reduced from onset (time 1) to discharge (time 2) and at home (time 3 and 4), but the improvement was greater in the intervention group. The overall score of the quality of life improved in both groups but to a greater extent in the intervention group. This also applies to the individual subgroups of quality of life. All results were statistically nonsignificant. Further details are given in Table 3.
Nurses outcome
A total of 24 nurses had participated in the study, 21 of whom were women. The average age was 40 years, and the professional experience in rehabilitation was seven years. There were 17 qualified nurses, six nursing assistants, and one person in training as a registered nurse. The knowledge score at the start of the control phase was 12.9 (standard deviation (SD) 2.92) out of 21 points; in the 2nd measurement, which took place between the end of the control phase and before the training, the score was 13.0 (SD 2.15); after the intervention phase, the score was 14.4 (SD 2.91); so the knowledge increased, but the difference was not significant.
Participants experience
In the interviews, participants talked about their difficulties in everyday life with urinary incontinence. They experienced incontinence as disturbing and unpleasant. They also reported feelings of “shame,” “disgust,” or “weakness” after incontinence incidents. They found it difficult to talk about this. Nevertheless, they found it pleasant to be asked about their problems regarding urinary incontinence during rehabilitation stay. It was appreciated if professionals took the time to talk about incontinence and incontinence materials in a separate room. All participants showed a willingness to treat their urinary incontinence and to learn and apply new behavioral interventions. Not all of them were able to remember the concrete measures retrospectively. Nevertheless, the five participating patients generally reported no deterioration in urinary incontinence as a result of the newly implemented incontinence management. Three out of five reported less urinary incontinence and general improvement. None of the participants felt that the interventions, which were individually tailored and implemented for them, were useless or experienced them negatively. Controlled drinking and toilet training were experienced as positive. They were motivated to continue using these behavioral interventions at home.
Discussion
In the current study, urinary continence as well as quality of life improved in the control and intervention groups; improvements were higher in the intervention group, but the differences were not significant. The knowledge of the nurses about urinary incontinence improved during the study duration. The results were nonsignificant. The acceptance of the intervention was high, and participants were motivated to continue with the individual adopted measures. Some of the participants experienced an improvement in the continence situation.
Regarding to McDonald et al. (2017), bladder training is advocated as the first-line intervention in the management of urge incontinence / overactive bladder in the general population. This effectiveness was examined in a randomized controlled trial by Vaughan et al. (2019) with 53 participants suffering from Parkinson’s disease. No studies could be found which investigated nonpharmacological interventions adopted to incontinence forms other than urge incontinence. Also in the review of Hajebrahimi et al. (2019), the two mentioned studies focused on pelvic floor training or bladder training. Some incontinence forms, for example, functional incontinence, were not addressed with these measures. Therefore, the present study is probably the first which examined individuals who adopted nonpharmacological measures in promoting continence in Parkinson’s disease including toilet training.
Fluid management was part of the intervention in the study of Vaughan et al. (2019) and also in the current study. Participants found that fluid restrictions in the evening are helpful. A Cochrane review of Imamura et al. (2015) could identify a small amount of very low-quality evidence from the included studies that investigated volume of fluid intake which suggested that symptoms of urinary incontinence may reduce when fluid intake is reduced although there were side effects such headaches, constipation, and thirst. It seems important that fluid restrictions were implemented with caution about side effects and the wellbeing of the affected person although no side effects were observed in the current study.
In the intervention group, the measure to evaluate the incontinence material was conducted in six cases. It was expected that this measure will be performed in more cases because in practice they observe many uncertainties among nurses and patients. Few studies could be found about this issue, and little is known about how the optimal management and election of optimal material should be performed (Fader, Cottenden & Getliffe, 2008; Miget et al., 2018), which means that there is a need for further research.
In the current study, the knowledge of nurses about urinary incontinence did not increase significantly. Ostaszkiewicz, Tomlinson and Hunter (2020) conducted a systematic review to search about the effects of education about urinary incontinence on nurses’ and nursing assistants’ knowledge and attitudes toward urinary incontinence, their continence care practices, and patient outcomes. They found mixed evidence, while nonrandomized and before-after studies tended to report positive effects. Results were not confirmed in RCTs. The authors concluded that further attention should be paid to improving methods to increase nurses’ ability to accurately identify patients’ frequency and severity of urinary incontinence. This requirement was fulfilled in the present study by implementing an incontinence assessment with precise specifications. The challenge for the further development of the intervention is not only the correct assessment but also the next step: how could the correct measures be identified based on the assessment, how could an algorithm help, and how could individual adopted measures be brought into a logical and comprehensible process?
Limitations
Several limitations of this study should be mentioned. First is the omission of randomization. The intervention was implemented in one Parkinson’s ward. Therefore, randomization, for example, cluster randomization was not possible. Another limitation is the recruiting phase. It took much longer than planned even though the sample was estimated based on past case numbers. Several reasons were discussed concerning this problem, and it is not entirely clear why the gap between the calculated number of persons recruited and the effective recruited participants was so great. Consequently, the sample size of this study was smaller than expected. Data could not be recorded completely in every case. Reasons for this were, for example, that the patients had not filled out a questionnaire correctly or that they had to be transferred due to deterioration of their condition. And due to multiple testing, a cumulative alpha error cannot be completely ruled out.
Conclusion
In the current clinical trial, a structured continence management program with individual adopted measures was tested in rehabilitation practice to promote urinary continence in persons suffering from PD. Improvements in incontinence symptoms and quality of life were measured in both control and intervention groups. The differences between admission and rehabilitation stay were greater in the intervention group. It is possible that the sample was too small to show significant differences. Acceptance of the intervention seems to be a given. The persons concerned were able to carry out the measures. The knowledge of the nursing staff regarding incontinence could be increased by the intervention. To our knowledge, this is the first study that has tested customized interventions such as toilet training in people with PD. The results are promising, and the intervention can now be further tested in a larger study with a robust design.
Relevance to clinical practice
In practice, it is important to perform a detailed assessment to identify problems regarding urinary incontinence. The most important thing, however, seems to be that nursing staff necessarily seek dialogue with the persons concerned. The nurse must take the initiative; otherwise, the patients would not address the issue on their own initiative. However, they are open to interventions and motivated to improve their situation with non-pharmacological interventions.
The authors would like to thank Denise Junker and Nadja Schweizer for their assistance in the study.
Prof. Dr. Myrta Kohler, Eastern Switzerland University of Applied Sciences, Rosenbergstrasse 59, 9001 St. Gallen, Switzerland, myrta.
What was the biggest challenge in your study?
Nursing staff training and data collection were intensive work packages in the project.
How could the topic be developed in the future?
Further research is required in the area of continence promotion in neurorehabilitation so that nursing practice can be sustainably improved.
What further reading do you recommend?
Funnell, S.C. and Rogers, P. J. (2011). Purposeful program theory: effective use of theories of change and logic models. San Francisco: Jossey-Bass / Wiley.

References
(2016). The epidemiology of Parkinson’s disease: Risk factors and prevention. The Lancet. Neurology, 15(12), 1257 – 1272.
(2004). Iciq: A brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourology and Urodynamics, 23(4), 322 – 330.
(2009). Rehabilitation nurses practices in relation to urinary incontinence following stroke: A cross-cultural comparison. Journal of Clinical Nursing, 18(7), 1049 – 1058.
(2017). “It comes and cannot be prevented” - Experiencing and coping with urinary incontinence in people with Parkinson’s syndrome. QuPuG – Journal Für Qualitative Forschung in Pflege- Und Gesundheitswissenschaft, 4(1), 16 – 23.
(2017). Evidence-Based Continence Care: An Integrative Review. Rehabilitation Nursing : The Official Journal of the Association of Rehabilitation Nurses, 42(6), 301 – 311.
(2004). Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: The TREND statement. American Journal of Public Health, 94(3), 361 – 366.
(2009). Prompted voiding for the management of urinary incontinence in adults (Review). Cochrane Database of Systematic Reviews, CD002113.
(2008). Absorbent products for moderate-heavy urinary and / or faecal incontinence in women and men. Cochrane Database of Systematic Reviews, CD007408.
(2019). Management of neurogenic bladder in patients with Parkinson’s disease: A systematic review. Neurology and Urodynamics, 38(1), 31 – 62.
(2007). How common are the “common” neurologic disorders? Neurology, 68(5), 326 – 337.
(1967). Parkinsonism: Onset, progression and mortality. Neurology, 17(5), 427 – 442.
(2014). Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ, 348, g1687.
(2015). Lifestyle interventions for the treatment of urinary incontinence in adults. Cochrane Database of Systematic Reviews, (Online)(12), CD003505.
(2000). Mini-mental-status-test (MMST). Göttingen: Beltz Test GMBH.
(2010).
Assessment der Lebensqualität von Patienten mit Harninkontinenz: Incontinence Quality of Life Instrument . In B. ReuschenbachC. MahlerEds., Pflegeassesment. Pflegebezogene Assessmentverfahren: Internationales Handbuch für Pflegeforschung und -praxis (pp. 582 – 584). Bern: Huber.(2015). Qualitative Inhaltsanalyse: Grundlagen und Techniken (12. überarb. Aufl.). Weinheim: Beltz.
(2017). Lower urinary tract symptoms in Parkinson’s disease: Prevalence, aetiology and management. Parkinsonism & Related Disorders, 35, 8 – 16.
(2018). Urinary Incontinence in neurological disease – Prevalence and influencing factors in patients of a rehabilitation clinic. Pflegezeitschrift, 71(5), 53 – 58.
(2018). Place des protections dans la prise en charge de l’incontinence urinaire [Absorbent products for urinary incontinence management]. Progres en urologie : journal de l’Association francaise d’urologie et de la Societe francaise d’urologie, 28(17), 953 – 961.
National Health Service . (2019). Non-surgical treatment – Urinary incontinence. Retrieved from https://www.nhs.uk/conditions/urinary-incontinence/treatment/ [August 25, 2021].(2010a). Habit retraining for the management of urinary incontinence in adults. Cochrane Database of Systematic Reviews, CD002801.
(2010b). Timed voiding for the management of urinary incontinence in adults. Cochrane Database of Systematic Reviews, CD002802.
(2020). The Effects of Education About Urinary Incontinence on Nurses’ and Nursing Assistants’ Knowledge, Attitudes, Continence Care Practices, and Patient Outcomes. Journal of Wound, Ostomy and Continence Nursing, 47(4), 365 – 380.
(1999). Cultural adaptation of a quality-of-life measure for urinary incontinence. European Urology, 36(5), 427 – 435.
(2019). Parkinson’s Disease. The Medical Clinics of North America, 103(2), 337 – 350.
(2008). Nurses’ knowledge and practice about urinary incontinence in nursing home care. Nurse Education Today, 28(8), 926 – 934.
(2013). Health-related quality of life and economic impact of urinary incontinence due to detrusor overactivity associated with a neurologic condition: a systematic review. Health and Quality of Life Outcomes, 11(1), 13.
(2019). Behavioral therapy for urinary symptoms in Parkinson’s disease: A randomized clinical trial. Neurourology and Urodynamics, 38(6), 1737 – 1744.
(2004). Bladder training for urinary incontinence in adults. Cochrane Database of Systematic Reviews, (Online)(1), CD001308.
(2015). Lower urinary tract dysfunction in patients with parkinsonism and other neurodegenerative disorders. Handbook of Clinical Neurology, 130, 335 – 356.



