Improvement of Cognitive Function after Physical Movement Training in Institutionalized Very Frail Older Adults with Dementia
Abstract
Physical exercise has positive effects on cognitive functioning in both healthy older adults and ambulatory older adults with dementia. The present study investigated whether a 10-week multimodal movement intervention conducted in the seated position can slow cognitive deterioration in demented and physically very frail nursing-home residents. Our analysis revealed that training participants showed no further overall cognitive deterioration throughout the study and a significant improvement in the ADAS-Cog orientation/praxis subscore (p = .04). In contrast, the control group demonstrated a significant decline in the ADAS-Cog sum score (p = .02). These results might be of relevance for geriatric practice since they indicate that a short-term physical intervention – even in the seated position – can decelerate cognitive decline and dementia despite physical frailty.
Introduction
The number of older citizens is ever growing, both in absolute and relative terms. Increasing age is the main risk factor of cognitive decline and dementia (e.g., Jorm & Jolley, 1998; Yaffe et al., 2009). Persons affected by dementia suffer from significant and progressive cognitive decline and increasing disturbances in social functioning and other activities of daily living. So far, there is no approach to prevent, treat, or cure dementia that has proven effective. However, recent research has shown that physical activity may be able to reduce the risk and to decelerate the progression of neurodegenerative processes (e.g., Heyn, Abreu, & Ottenbacher, 2004; Lautenschlager et al., 2008).
Healthy and physically active older adults show significantly better cognitive function and less cognitive decline (Lautenschlager & Almeida, 2006). Regular physical activity (e.g., walking, bicycling, hiking, or swimming at least three times a week) may reduce the risk or delay the onset of dementia (Abbott et al., 2004; Hertzog, Kramer, Wilson, & Lindenberger, 2008; Larson et al., 2006; Stewart, Richards, Brayne, & Mann, 2001). In people who carry the genetic risk factor apoliopoprotein ApoE ε4 allele, which is associated with an enhanced risk of developing dementia, the positive effects of an active lifestyle (i.e., more than one hour of physical activity throughout the day) seem to be even more pronounced (Schuit, Feskens, Launer, & Kromhout, 2001). Physical fitness training improves cognitive and especially executive functions of healthy but otherwise sedentary older adults (Colcombe & Kramer, 2003). Furthermore, physical fitness training is associated with a significant increase in task-related brain activity in the attentional network and increased gray and white matter volume and may therefore counteract normal age-related losses of brain tissue and function (Colcombe et al., 2004, 2006; Erickson et al., 2011). In a meta-analytic review, Smith et al. (2010) revealed improvements in executive function, attention, speed of processing, and memory in healthy older adults and adults with mild cognitive impairment (MCI) after aerobic training compared to control groups with nonaerobic exercise. In a randomized controlled study by Lautenschlager et al. (2008), older adults at risk of developing dementia (i.e., with subjective memory complaints, with MCI, or carrying the genetic risk factor ApoE ε4 allele) were assigned to either a 24-week physical fitness program or to an education and treatment-as-usual control group. Participants in the intervention group improved their cognitive function by .26 points on the Alzheimer Disease Assessment Scale – Cognitive Subscale (ADAS-Cog) compared to a deterioration of 1.04 points in the treatment-as-usual group. The importance of this finding is of course not the minimal improvement but the retardation of further decline when physical exercise is introduced.
In a meta-analysis, Heyn et al. (2004) analyzed 30 randomized trials, altogether 2,020 participants with dementia, investigating the effect of physical fitness training against control conditions. Although most of the studies included had been conducted in nursing homes, long-term care residencies, or residencies for older people, most of the exercises consisted of walking or combined walking and isotonic (dynamic muscle strengthening) training. The meta-analysis revealed no significant training effect × training characteristics interaction. Nevertheless, the authors concluded that physical exercise training does improve health-related physical fitness as well as cognitive function of older adults with dementia, though they did not recommend an optimum type or minimum duration of the training. However, to our knowledge, results of the Heyn et al. meta-analysis cannot be generalized to physically very frail older adults with dementia (see also Forbes et al., 2008; Lautenschlager, Almeida, Flicker, & Janca, 2004). In addition, more recent randomized controlled studies show rather mixed results concerning the potential effects of physical exercise on the cognitive functions of institutionalized people with dementia. For example, Kemoun et al. (2010) found significant improvements in overall cognitive functions in older but physically unimpaired nursing home residents with moderate to severe Alzheimer’s disease after 15 weeks of physical training (mainly walking, stamina, and balance exercises) compared to a control group. However, Eggermont, Swaab, Hol, and Scherder (2009) did not find a significant time × group interaction for older nursing home residents with moderate dementia.
The obvious need for an effective approach to decelerating cognitive deterioration and enhancing the quality of life of nursing-home residents with progressive dementia and physical impairments is a current matter of debate among concerned relatives, scientists, medical professionals, and health insurance companies. In particular, the present literature tends to focus on older adults who are still somewhat mobile, which may miss the reality of sufferers from dementia, who often need to rely on walking aids or wheelchairs.
The present study investigates the potential benefits of a moderate-intensity multimodal movement program for institutionalized and very frail patients with dementia. The analysis of the training effects focused on cognitive function, mood, and activities of daily living.
Method
Participants
A controlled study was conducted in two nursing home residencies of the community health organization “Spitalstiftung” in Konstanz, Germany. Both were comparable with respect to living conditions, and both offered the same treatment-as-usual protocols. A total of 32 volunteers were screened for cognitive and physical eligibility. Figure 1 shows the number of volunteers at screening and the number of participants completing the posttest phase. We only included older adults with a record of dementia who were physically frail but cognitively and physically eligible for participating in the neuropsychological and physical examinations as well as in the physical movement training. Exclusion criteria comprised the following: no indication of dementia (according to DSM-IV-TR criteria; MMSE > 24, range 0–30), salient behavioral problems or lack of minimally sufficient daily functioning, severe sensory impairments, absence of or severe impairments in written or spoken German and lack of minimal physical eligibility (incl. hemiplegia and paraplegia). Finally, volunteers who had already taken part in the gymnastic group of the nursing homes were also excluded. 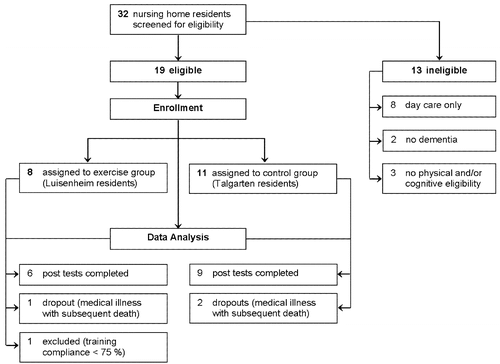
A total of 19 nursing home residents took part in the controlled trial. Since group assignment depended on nursing-home residency for logistical reasons, 8 residents of the Luisenheim formed the training group and 11 residents of the Haus Talgarten formed the treatment-as-usual control group. All participants relied partly or constantly on walking aids. Six participants of the training group (age range 74–92 years, 4 women) and 9 participants of the control group (age range 82–92 years, 7 women) were included in the statistical analysis (i.e., completed pre- and postassessments, attended training at least at a 75% rate). All participants suffered from dementia according to the DSM-IV-TR criteria (mean MMSE = 17.3, SD = 4.55, range 5–24). Both groups showed similar distributions of male and female participants (χ2(df = 1) = .23, p = .63) and did not significantly differ in their educational level. Because of their age and state of health, all participants were taking some medication (most commonly against hypertension).
The Ethics Committee of the University of Konstanz, Germany approved this study. Written informed consent was obtained from the participants and their legal guardians before participation. After study completion, physical movement training was also offered to the control participants.
Procedure
Selected participants were allocated either to the movement intervention or waiting list control group (usual care) depending on the nursing home of residency. Accordingly, participants and research personnel could not be blinded to group membership. Randomized allocation to groups was not possible as participants were not able to visit the other building for training because of their physical frailty.
The pre- and postassessments of cognitive functions were carried out by experienced psychologists or psychology master students. The physical fitness tests as well as the physical movement training sessions were instructed by four experienced sports master students (2 males, 2 females; 1 male and 1 female per session). The rather high trainer-to-participant ratio was chosen in order to ensure complete safety throughout the training sessions. All responsible students were under close supervision of experienced psychologists or sports majors. Selected nursing home staff members were always available during testing and training sessions in case of emergency.
Instruments
Assessment of Physical Fitness
Three different physical fitness tests, adequate for frail nursing home residents, were applied before the intervention in order to assess initial physical capabilities. The chair-rise test (e.g., Bohannon, 1995) assesses the strength of the lower extremities; the functional-reach test (e.g., Duncan, Weiner, Chandler, & Studenski, 1990) measures balancing ability in daily tasks; and the get-up-and-go test (e.g., Bös, 2001) also measures balance during daily activities and gives additional information concerning risk of falls. Throughout the physical fitness testing, all participants used their walking aids. Due to the cognitive impairments of the participants, it was not possible to assess lifetime physical activity retrospectively.
Assessment of Cognitive Function, Mental Health, and Activities of Daily Living
Overall dementia screening was conducted using the Mini Mental State Examination (MMSE, German version; e.g., Folstein, Folstein, & McHugh, 1975). MMSE scores of 24 points or less (out of 30) indicate cognitive impairments or dementia. Further evaluation of dementia followed the DSM-IV-TR criteria (American Psychiatric Association [APA], 2000).
As primary outcome measure we used the Alzheimer Disease Assessment Scale – Cognitive Subscale (ADAS-Cog, German version; e.g., Ihl & Weyer, 1993), form A for pretests and parallel form B for posttests. The scale consists of three subscales: memory (range 0–22 errors), orientation/praxis (range 0–28 errors), language (range 0–15 errors), and one additional item on the ability of recollecting test instructions (total ADAS-Cog error score range 0–70). Cognitive assessment was complemented using a modified CERAD-Plus version (The Consortium to Establish a Registry for Alzheimer’s Disease, German revised edition; Memory Clinic Basel, 2005; Welsh, Butters, Mohs, & Beekly, 1994): subtests verbal fluency, phonematic fluency, and Trail Making Tests (TMT) A and B for more detailed assessment of executive functions and speed of processing.
We monitored depression symptoms during pre- and posttest phases using the Geriatric Depression Scale-15 (GDS-15; 15-item short German version; Gauggel & Birkner, 1999; Yesavage et al., 1982; range 0–15 points).
Daily functioning was assessed using the Alzheimer’s Disease Functional Assessment and Change Scale, which is sensitive to possible change in clinical trials (ADFACS; Burns, Lawlor, & Craig, 2002; Galasko et al., 1997). The scale allows evaluation of individual competence in ten instrumental activities of daily living (IADL) in geriatric patients (range 0–30 points). Most relevant, the questionnaire also involves six basic activities of daily living (ADL; range 0–24 points). Higher ADFACS scores indicate more severe impairments of IADL and ADL. The scale was administered as observer-rating scale (nursing staff rating).
Physical Movement Intervention
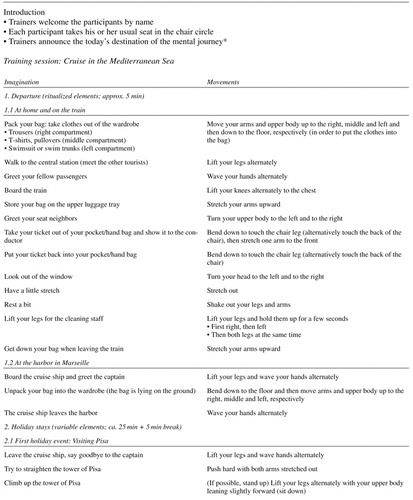
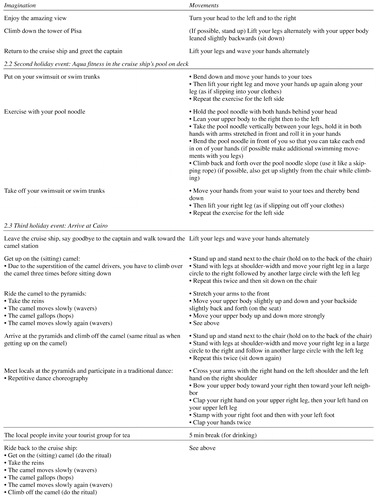
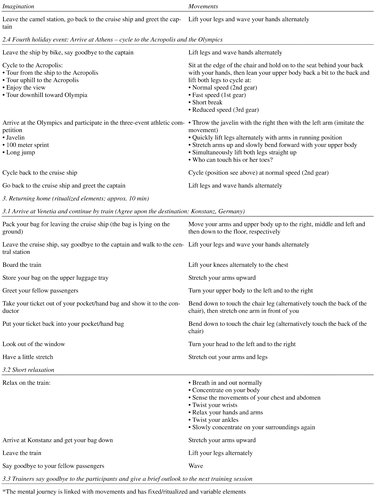
Participants of the intervention group continued their standard daily activities and classes but received additional moderate-intensity multimodal physical movement training. Training took place twice a week for 45 minutes each. Due to general physical frailty and need for walking aids of the participants, physical exercises were mainly conducted in a seated position but gradually increased in level of difficulty and complexity. The training combined strengthening, coordination, balance, flexibility, and stamina. All exercises were embedded in a context of a mental journey in order to increase motivation, commitment, and pleasure (see also AppendixTable 1 for a sample training session). Additionally, the training included repeated memory aids to focus attention and keep track of the exercises (e.g., “Where are we?” – “What are we supposed to do next?”). Accordingly, this physical movement intervention utilized the social character of the group intervention in order to enhance training adherence. Stimulation by music or singing was not included.
Treatment-As-Usual Control Group
Participants of the treatment-as-usual control group took part in standard care activities of the community health organization Spitalstiftung (except in the gymnastic class of the nursing homes). These activities included handicrafts, singing, playing music, movie afternoons, cooking and baking together, going for a walk together, and participating in festivals and memory trainings. Furthermore, the nursing homes organized regular church services and family days. The nursing home staff motivated the residents to stick to their daily schedule.
Daily activities of the control and the intervention group followed the same treatment-as-usual protocol of the same community health organization.
Data Analysis
Statistical analysis was done using the R statistical software package of The R Foundation of Statistical Computing (www.R-project.org; version 2.11.1 for Mac OS X, GUI 1.34 Leopard).
Because of the small sample size, sample characteristics and neuropsychological variables at baseline were compared between groups using the nonparametric Exact Wilcoxon rank sum test (W; package exactRankTests for R; Hothorn & Hornik, 2010). For categorical variables Pearsons’s chi-squared (χ2) tests were computed. Mixed effects analysis of variance models with a random intercept for participants were calculated in order to analyze potential training effects (package nlme for R; Pinheiro, Bates, DebRoy, Sarkar, & the R Development Core Team, 2011). Normality assumptions of the model residuals were tested using the Shapiro-Wilk normality test. The F-statistic of the corresponding posthoc tests is robust with respect to nonnormal (even strongly kurtotic or skewed) distributions, and type-I error rate is negligible even in very small sample sizes (Stevens, 2002). All tests for statistical significance were two-tailed and referred to a significance level with α = .05.
Results
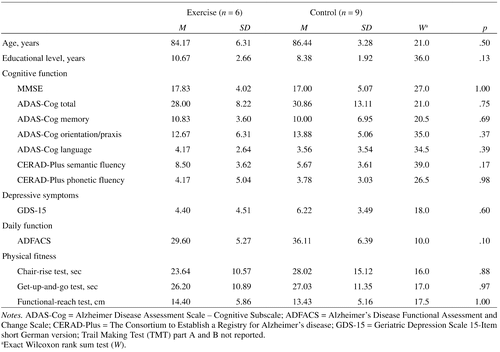
Sample characteristics are depicted in Table 1. The two groups did not differ according to demographic variables, physical fitness, daily functioning, depressive symptoms, or cognitive function at baseline. Test scores of the Trail Making Test (TMT) A and B were excluded from data analyses since most participants were not able to complete the tests.
Participants of the intervention group took part in at least 75% of all 20 training sessions (M = 18.67 sessions, SD = 1.83, range 15–20). Only one out of eight participants of the initial training group had to be excluded from data analysis due to insufficient training compliance of only 40% (see also Figure 1).
Effects of the Physical Intervention on Cognitive Function
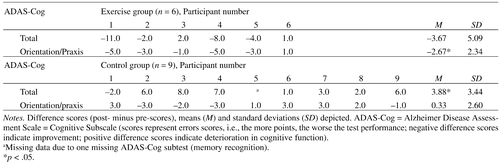
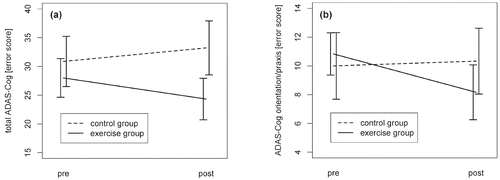
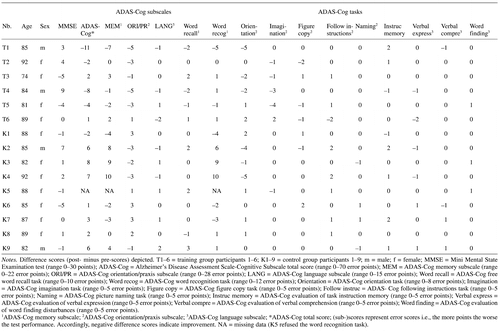
Mixed effects models revealed a significant Group × Time interaction for the total ADAS-Cog score (F[1, 12] = 10.76, p = .007) and the ADAS-Cog orientation/praxis subscore (F[1, 13] = 5.18, p = .04; see Figure 2). Posthoc tests revealed significant deterioration of the control participants of 3.9 error points on average (SD = 3.44) in the total ADAS-Cog score over time (F[1, 7] = 9.91, p = .02), whereas the intervention group did not show a significant change (F[1, 5] = 3.12, p = .14; see Figure 2a) but had a tendency toward improvement with on average –3.7 error points (SD = 5.09). In the ADAS-Cog orientation/praxis subscore, the intervention group showed significant improvements of on average –2.7 error points (SD = 2.34) over time (F[1, 5] = 7.80, p = .04), whereas the control group did not (change: M = .3 error points, SD = 2.60; F[1, 8] = .15, p = .71; see Figure 2b). Considering age as a potential covariate in the mixed effects models did not change the reported results. (See also AppendixTable 2 for the individual change scores.)
Effects of the Intervention on Depression and Activities of Daily Living
There were no significant interactions or main effects for depressive symptoms (GDS-15) or daily function (ADFACS).
Discussion
The above results show that a short and multimodal physical movement training of only 10 weeks – even when exercise is conducted solely in the seated position – has positive effects on cognitive function in physically very frail nursing home residents with dementia.
Consistent with previous findings, this study confirms the expected influence of physical training on cognition in ambulatory older adults and ambulatory people with dementia: In healthy older adults and older adults with MCI, physical fitness training has known positive effects on cognitive function, and beneficial effects are largest when aerobic exercise is multimodally combined with strength and flexibility components (e.g., Colcombe & Kramer, 2003; Lautenschlager et al., 2008; Smith et al., 2010). Positive effects of physical training are also observed in people with dementia who are otherwise still ambulatory (e.g., Heyn et al., 2004; Kemoun et al., 2010). However, the few randomized controlled studies conducted to date offering a physical intervention in seated position to very frail participants with dementia (omitting aerobic training components as well as stimulation by music or singing) either did not focus on cognitive outcomes (Lazowski et al., 1999; Schnelle et al., 1996) or did not report possible cognitive changes (Schnelle et al., 1996). The present study extends these previous findings by showing the beneficial effects of a physical intervention on cognition even in physically very frail older adults with dementia.
The major limitations of the present study include the small number of participants and the group assignment according to the site of residency. However, both residencies belonged to the same community health provider (Spitalstiftung) and followed the same treatment-as-usual protocol, so that potential influences of location were at least minimized. Both groups showed a tendency toward differences in level of education, semantic fluency, and daily functioning (ADFACS) at baseline, which did not reach statistical significance (see also Table 1). However, such variables should be better matched between groups in future work with larger sample sizes since they might have an influence on the training outcomes. Furthermore, because of the multimodal character of the physical intervention, it is also not yet clear which components mainly account for the reported training effects. However, this question was not the aim of the present study and remains to be addressed in future research. Finally, the present study also gives no information about the long-term stability of the observed cognitive effects since we did not conduct follow-up assessment of 3 months or more. This question should also be addressed in future studies.
In summary, despite the study’s limitations, our results provide first indications that even very frail people with dementia can benefit from physical exercise. Despite the small sample size, the short-term and nonaerobic but multimodal character of the intervention, participants showed significant improvements in orientation and praxis compared to controls. Furthermore, training participants showed preservation of overall cognitive function, whereas control participants showed further significant cognitive decline. It is also important to note that overall training compliance was high among participants despite their physical and cognitive limitations (only one participant had to be excluded due to insufficient training compliance below 75%). The present findings are very encouraging for future research and might be of relevance for geriatric practice. Randomized controlled studies with larger age- and gender-matched samples are needed in order to confirm and further elaborate these findings.
Conflicts of Interest
None.
References
(2004). Walking and dementia in physically capable elderly men. Journal of the American Medical Association, 292, 1447–1453. doi: 10.1001/jama.292.12.1447
(2000). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: Author.
(1995). Sit-to-stand test for measuring performance of lower extremity muscles. Perceptual and Motor Skills, 80, 163–166. Retrieved from www.ncbi.nlm.nih.gov/pubmed/7624188
(2001). Handbuch motorische Tests: Sportmotorische Tests, motorische Funktionstests, Fragebogen zur körperlich-sportlichen Aktivität u. sportpsychologische Diagnoseverfahren [
Handbook of motor tests: sport-motor tests, motor function tests, questionnaires on physical activities and sport psychological diagnostic tools ] (2nd ed.). Göttingen: Hogrefe.(2002). Rating scales in old age psychiatry. The British Journal of Psychiatry, 180, 161–167. Retrieved from www.ncbi.nlm.nih.gov/pubmed/11823329
(2003). Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science, 14, 125–130. Retrieved from www.ncbi.nlm.nih.gov/pubmed/12661673
(2006). Aerobic exercise training increases brain volume in aging humans. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 61, 1166–1170. Retrieved from www.ncbi.nlm.nih.gov/pubmed/17167157
(2004). Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences of the United States of America, 101, 3316–3321. doi: 10.1073/pnas. 0400266101
(1990). Functional reach: A new clinical measure of balance. Journal of Gerontology, 45, M192–7. Retrieved from www.ncbi.nlm.nih.gov/pubmed/2229941
(2009). Walking the line: A randomized trial on the effects of a short term walking program on cognition in dementia. Journal of Neurology, Neurosurgery, and Psychiatry, 80, 802–804. doi: 10.1136/jnnp. 2008.158444
(2011). Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America, 108, 3017–3022. doi: 10.1073/ pnas.1015950108
(1975). “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. Retrieved from www.ncbi.nlm.nih.gov/pubmed/1202204
(2008). Physical activity programs for persons with dementia. Cochrane Database of Systematic Reviews (Online), (3), CD006489. doi: 10.1002/14651858.CD006489.pub2
(1997). An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Disease and Associated Disorders, 11(Suppl. 2), S33–39. Retrieved from www.ncbi.nlm.nih.gov/pubmed/9236950
(1999). Validität und Reliabilität einer deutschen Version der Geriatrischen Depressionsskala (GDS) [
Validity and reliability of a German version of the Geriatric Depression Scale (GDS) ]. Zeitschrift für Klinische Psychologie, 28, 18–27. doi: 10.1026//0084–5345.28.1.18(2008). Enrichment effects on adult cognitive development: Can the functional capacity of older adults be preserved and enhanced? Psychological Science in the Public Interest, 9, 1–65. Retrieved from www.redi-bw.de/db/ebsco.php/search.ebscohost.com/login.aspx?direct=true&db=psyh&AN =2009–08269–002&site=ehost-live
(2004). The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Archives of Physical Medicine and Rehabilitation, 85, 1694–1704. Retrieved from www.ncbi.nlm.nih.gov/pubmed/15468033
(2010). exactRankTests: Exact Distributions for Rank and Permutation Tests. R package version 0.8–19. Retrieved from cran.r-project.org/package=exactRankTests
(1993). Die Alzheimer Disease Assessment Scale (ADAS) [
Alzheimer Disease Assessment Scale (ADAS) ]. Weinheim: Beltz Test.(1998). The incidence of dementia: A meta-analysis. Neurology, 51, 728–733. Retrieved from www.ncbi.nlm.nih.gov/pubmed/9748017
(2010). Effects of a physical training program on cognitive function and walking efficiency in elderly persons with dementia. Dementia and Geriatric Cognitive Disorders, 29, 109–114. doi: 10.1159/000272435
(2006). Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Annals of Internal Medicine, 144, 73–81. Retrieved from www.ncbi.nlm.nih.gov/pubmed/16418406
(2006). Physical activity and cognition in old age. Current Opinion in Psychiatry, 19, 190–193. doi: 10.1097/01.yco.0000214347.38787.37
(2004). Can physical activity improve the mental health of older adults? Annals of General Hospital Psychiatry, 3, 12. doi: 10.1186/1475-2832-3-12
(2008). Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: A randomized trial. Journal of the American Medical Association, 300, 1027–1037. doi: 10.1001/jama.300.9. 1027
(1999). A randomized outcome evaluation of group exercise programs in long-term care institutions. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 54, M621–M628. Retrieved from www.ncbi.nlm.nih.gov/pubmed/10647968
(2011). nlme: Linear and nonlinear mixed effects models. R package version 3.1–101. Retrieved from cran.r-project.org/web/packages/nlme/citation.html
(1996). Exercise with physically restrained nursing home residents: Maximizing benefits of restraint reduction. Journal of the American Geriatrics Society, 44, 507–512. Retrieved from www.ncbi.nlm.nih.gov/pubmed/8617897
(2001). Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Medicine and Science in Sports and Exercise, 33, 772–777. Retrieved from www.ncbi.nlm.nih.gov/pubmed/11323547
(2010). Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosomatic Medicine, 72, 239–252. doi: 10.1097/PSY.0b013e3181d14633
(2002). Applied multivariate statistics for the social sciences (4th ed.). Mahwah, NJ: Erlbaum.
(2001). Vascular risk and cognitive impairment in an older, British, African-Caribbean population. Journal of the American Geriatrics Society, 49, 263–269. Retrieved from www.ncbi.nlm.nih.gov/pubmed/11300236
(1994). The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): V. A normative study of the neuropsychological battery. Neurology, 44, 609–614. Retrieved from www.redi-bw.de/db/ebsco.php/search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=1994–35821–001&site=ehost-live
(2009). Predictors of maintaining cognitive function in older adults: The Health ABC study. Neurology, 72, 2029–2035. doi: 72/23/2029 [pii] 10.1212/WNL. 0b013e3181a92c36
(1982). Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research, 17, 37–49. Retrieved from www.ncbi.nlm.nih.gov/pubmed/7183759
This research was funded by the Heidelberg Academy of Sciences and Humanities, Heidelberg, Germany, and the Zukunftskolleg of the University of Konstanz, Konstanz, Germany.


