Social Psychology and Noninvasive Electrical Stimulation
A Promising Marriage
Abstract
Abstract. Social neuroscience and psychology have made substantial advances in the last few decades. Nonetheless, the field has relied mostly on behavioral, imaging, and other correlational research methods. Here we argue that transcranial direct current stimulation (tDCS) is an effective and relevant technique to be used in this field of research, allowing for the establishment of more causal brain-behavior relationships than can be achieved with most of the techniques used in this field. We review relevant brain stimulation-aided research in the fields of social pain, social interaction, prejudice, and social decision-making, with a special focus on tDCS. Despite the fact that the use of tDCS in Social Neuroscience and Psychology studies is still in its early days, results are promising. As better understanding of the processes behind social cognition becomes increasingly necessary due to political, clinical, and even philosophical demands, the fact that tDCS is arguably rare in Social Neuroscience research is very noteworthy. This review aims at inspiring researchers to employ tDCS in the investigation of issues within Social Neuroscience. We present substantial evidence that tDCS is indeed an appropriate tool for this purpose.
Social neuroscience is a prominent topic in contemporary psychology. Here, we present a review of studies using transcranial direct current stimulation (tDCS) to investigate social cognition. In this review, we aimed to describe how and why the use of tDCS can foster the development of social neuroscience and psychology. This technique allows for the safe and effective direct modulation of ongoing brain activity in human beings and the establishment of causality in brain-behavior relationships that cannot be achieved with imaging or behavioral methods alone. Despite the reduced number of studies using tDCS as a tool to understand social phenomena, we believe that it should be included in the social neuroscience toolkit. We will present recent studies providing preliminary evidence that this technique can help the social psychologist and neuroscientist to foster knowledge on social pain, empathy, social decision-making, and prejudice.
One main human characteristic is a social nature, given a high dependence on one’s peers to survive and on their welfare (Brent, Chang, Gariépy, & Platt, 2014; DeVries, Glasper, & Detillion, 2003). Although clinical studies with brain-lesioned patients have already shed light on some of the structural and functional aspects of the brain related to the processing of social information (e.g., Damasio, Grabowski, Frank, Galaburda, & Damasio, 1994), only in recent decades has the field developed to the point of systematically investigating the intricate neural processing of social information. This was possible due to recent technological advances (e.g., more computational power, new data collection techniques, new statistical methods), as well as the accumulation and interchange of knowledge from diverse areas such as natural and social sciences. An important result of this evolution is the germination of social neuroscience (Lieberman, 2007).
Social neuroscience originated by the merging of methods and knowledge from neuroscience, social psychology, and social sciences. Its main objective is to understand the neurobiological bases of social phenomena, aiming to investigate how human beings and other animals perceive themselves, perceive others, and how they interact (Lieberman, 2007). Although the origin of social neuroscience can be related to studies conducted in the late 20th century that aimed to investigate the biological basis of social processes (e.g., Cacioppo & Berntson, 1992), the major expansion and development of this field occurred in the last 20 years – one of the hallmarks of this expansion was the first social cognitive neuroscience meeting in 2001 (Lieberman, 2007).
In the next two sections, we will discuss the relevance of the correlational methods of social neuroscience and argue why and how noninvasive brain stimulation methods are of utmost importance in this field.
Advances Based on fMRI, EEG/ERP, Lesions
Before the introduction of the main noninvasive brain stimulation methods used today, most of the research on social cognition relied on behavioral methods, lesions, and/or correlational methods alone. Lesion studies represent some of the first investigations and demonstrations of specific roles of different brain areas in social cognition. Among these, there is no doubt that Phineas Gage is the most popular. After a lesion in the frontal cortex (more specifically, the bilateral prefrontal cortex), Phineas started to struggle with a number of cognitive and behavioral changes. In many textbooks, this case is discussed in terms of personality changes after the brain lesion (see Macmillan, 2008). Nonetheless, it is easy to argue that Phineas Gage was one of the first and most notable case studies of the role of the frontal lobe in social cognition. A lack of inhibitory control, impaired decision-making processes, emotional processing, and compliance with social norms are among the main changes observed in patients with similar lesions.
For a long time, lesion studies remained one of the few opportunities to investigate the role of the brain in social cognition until the popularization of electroencephalography (EEG) and other brain imaging techniques during the last two decades of the 20th century (for reviews of lesion studies in social cognition, see Bechara, 2004; Hillis, 2014). EEG has been used to understand social cognition in terms of its dynamics and timing of neural processing. Several event-related potentials (ERP) have been described throughout the last few decades and are significantly correlated with some specific tasks and processes. This technique has led to substantial advances in our understanding of social cognition processes. One of the first studies to use EEG to investigate social cognition evaluated the effects of one week of social isolation in healthy volunteers (Zubek, Bayer, & Shephard, 1969). These authors found no significant changes in subjective stress or intellectual tests but identified a significant decrease in alpha oscillations after the social isolation. This result highlighted one of the strengths of the brain imaging technique: to unveil implicit processes where no directly observable behavior can be identified. As examples of that, many authors have used EEG to study implicit attitudes (e.g., Amodio et al., 2004).
With limited temporal resolution but higher spatial resolution than EEG, functional magnetic resonance imaging (fMRI) facilitated the understanding of which brain areas are correlated with different types of social cognition demands. The contribution of fMRI to the neuroscience of social cognition has come a long way. Recently, its use as a diagnostic tool for some conditions with clear affective and social deficits has also been investigated. Just, Cherkassky, Buchweitz, Keller, and Mitchell (2014) found that a neurocognitive marker of autism could be identified in fMRI recordings and developed an algorithm that separated autistic patients from controls with 97% accuracy (Just, Cherkassky, Buchweitz, Keller, & Mitchell, 2014).
Lesions, EEG, and brain imaging studies have provided relevant discoveries about our social brain. However, several questions can only be answered by directly modulating the brain. In these cases, neuromodulation techniques may be used to probe causality in the brain-behavior relationship.
Neuromodulation techniques may be considered a revolution in modern neuroscience. The possibility to noninvasively and transiently interfere with the ongoing brain function using a site-specific technique allows us to understand brain-behavior relationships with another level of causality that cannot be achieved with the imaging of behavioral methods alone. It also represents an alternative to lesion studies because there are several limitations that can easily be overcome in brain stimulation studies. Among these, we highlight the following: (i) a lesion also leads to long-term adaptation and plasticity in other brain areas that might become a confounding factor for research, and (ii) researchers cannot control the place and size of lesions, and it is certainly not possible to implement repeated-measures designs comparing patients with and without the lesion (Pascual-Leone, Walsh, & Rothwell, 2000).
Neuromodulation Techniques
One of the most popular neuromodulatory techniques is TMS. It allows for the delivery of single or repetitive pulses to relatively superficial and focal (1 cm2) brain areas. For the purpose of this review, it is not important to discuss whether the induced effect is a facilitation or interference because the unique feature of TMS is its ability to interact transiently with the stimulated area of the brain, thus modifying the activity of that area and allowing one to evaluate its function (Miniussi, Harris, & Ruzzolid, 2013).
As the TMS technique became popular, it quickly started to play a central role in neuroscience research. From 1997 to 2007, the number of papers grew from less than 500 a year to approximately 2000 a year, and this number keeps growing. In the field of social cognition research and rehabilitation, a growing number of studies with TMS can also be seen. An estimation of the number of publications to mention the terms “Transcranial Magnetic Stimulation” and “Social” per year shows a similar growing trend, although the overall numbers are still arguably modest (peaking at approximately 250 papers a year, see Figure 1). The impact of TMS in social neuroscience and psychology might be exemplified by the study of Young, Camprodon, Hauser, Pascual-Leone, and Saxe (2010). Authors showed that TMS-induced disruption of the right temporal parietal junction (TPJ) affects moral judgment. In this study, participants had to judge how deserving of punishment were actors who tried to induce harm in innocent people. Participants receiving TMS to the TPJ were less reliable on the actor’s mental states and became more morally permissible (judging failed attempts to induce harm as less deserving of punishment than controls). These results clearly illustrate that TMS can affect social cognition in robust and very specific ways.
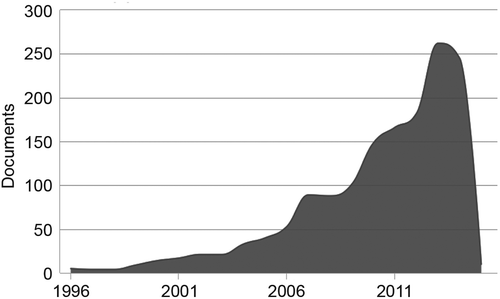
Therefore, if TMS is very effective in social cognition research, and because there is much more research using TMS than tDCS, why are we presenting a review of tDCS-aided social cognition studies? Similar to TMS, tDCS can allow the researcher to understand the role of one function in one specific area by observing in a repeated-measures design how increased versus decreased excitability of the area might affect behavior. However, when compared to TMS, tDCS did have a set of advantages that justified its use instead of TMS in many cases – particularly its use in the field of psychology outside medical environments. The main advantage is safety. Both procedures are reasonably safe when standard parameters are used, but rTMS can indeed induce seizures, and the field guidelines suggest that it should only be delivered in hospital environments (Rossi, Hallett, Rossini, & Pascual-Leone, 2009). tDCS has no such limitations and is painless, easy to deliver, highly portable, and low in cost (for a recent review, see Filmer, Dux, & Mattingley, 2014). It also has a more reliable placebo control (see Nitsche et al., 2008 for a discussion of this issue). It is associated with moderate and sparse adverse effects (Brunoni et al., 2011). Pain is not commonly observed during tDCS, whereas up to 40% of participants receiving TMS report pain, headache, or any other type of discomfort (Anderson et al., 2009; Loo, McFarquhar, & Mitchell, 2008). A study has shown that the discomfort caused by TMS could influence participants’ behavior and be a confounding variable (Abler et al., 2005). Despite the fact that it has not been investigated, TMS-caused discomfort could also be a confounding variable in social cognition studies, and in this case, tDCS would prove to be more reliable once its application was painless. Another tDCS advantage over TMS in social cognition is the easiness of use in social interaction studies with the application of simultaneous stimulation in two or more participants (Knoch et al., 2008). Finally, portability is also an interesting characteristic of tDCS. New studies might employ tDCS coupled with other portable tools (e.g., eyeglass) during social interactions outside the laboratory.
However, it is important to note that tDCS has some limitations when compared to TMS that might help to explain why this technique was not used more frequently in the field of social cognition. To understand these limitations, we need to provide a quick explanation of how tDCS works.
The most popular tDCS procedure consists of the application of low-intensity current (usually up to 2 mA) via two electrodes (anodal and cathodal) superficially positioned over the scalp. Different electrode sizes have been used, but the main electrode varies from 25 to 35 cm2. The current flows from the anode to the cathode. In general, the brain region below the anodal electrode presents an increase in excitability, while the opposite is observed for the cathodal electrode. This effect is attributed to a change in resting membrane potentials – differently from TMS, tDCS does not induce action potentials. TDCS also seems to induce longer lasting effects by “LTP-like” and “LTD-like” plasticity mechanisms (Stagg & Nitsche, 2011). Many works suggest that anodal tDCS may inhibit GABA (e.g., Nitsche et al., 2004), while cathodal stimulation inhibits glutamate (e.g., Stagg et al., 2009), a fact that can also help to explain the excitatory and inhibitory effects of anodal and cathodal stimulation. Because a detailed explanation of tDCS function and its mechanisms of action is beyond the scope of the present review, we refer to the recent reviews by Stagg and Nitsche (2011), Medeiros et al. (2012), and Filmer et al. (2014).
As described above, the electrode sizes were not small; therefore, one of the most crucial limitations is focality. tDCS is known to have a low spatial focality, as it uses large electrodes, and the current might spread beyond the targeted area (although recent current flow models suggest that most of the current density is concentrated below the electrodes and dissipates in between electrodes, see Wagner et al., 2014). In this scenario, targeting very small and specific areas may be challenging or even impossible. Nonetheless, whenever targeting large areas or cognitive processes that are not highly localized, low tDCS focality might be useful.
Another relevant issue is that both anodal and cathodal electrodes have functional effects. Thus, both electrodes will produce significant effects if located anywhere in the head (as is the case in most works in this field). Therefore, electrode placement could produce significant effects that might work as confounding factors (see Nitsche et al., 2008 for a discussion). In many cases, this could not be seen as a limitation. A particular study might benefit by observing how the simultaneous excitation and inhibition of different brain areas affects behavior (as Brunoni, Boggio, Ferrucci, Priori, & Fregni, 2013, for example). However, whenever the functional effects of the reference electrode are unwanted, some solutions are possible. Many studies have employed different electrode sizes of tDCS to minimize these effects (e.g., Fregni et al., 2008). Placing the reference electrode outside the head is a possible solution as well (e.g., Ferrucci et al., 2008). High-definition tDCS is another recent solution that seems to deliver more focal stimulation (see Edwards et al., 2013).
Lastly, tDCS has a very limited temporal resolution when compared with TMS. So whenever a high temporal resolution is needed, with very transient (even of the order of milliseconds) periods of stimulation, tDCS is certainly not the most adequate technique.
Nonetheless, we argue that tDCS’s safety, ease of use, cost, portability, and robust effects make a case for the more widespread use of this technique in contemporary psychology and neuroscience. In the next sections, we will review some of the most successful studies to use tDCS as a tool in social neuroscience and psychology research.
Social Neuroscience and Psychology Studies With Noninvasive Brain Stimulation and tDCS
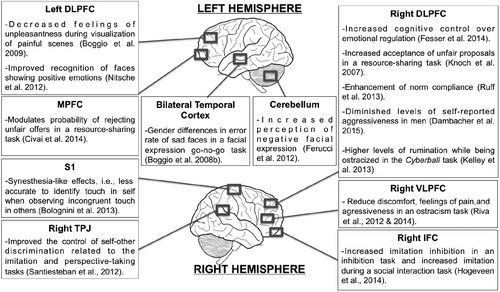
Here, we will review a few relevant topics in social neuroscience research that have employed noninvasive brain stimulation techniques successfully. We will focus on social pain, social interaction, prejudice, and social decision-making. Figure 2 depicts the target-areas and the main findings of each study on social neuroscience that are cited along the manuscript.
Social Pain and Touch, Empathy for Pain and Touch
Pain is a topic of great interest for social neuroscience and psychology. Typically, it is observed that structures linked to proprioception, conflict detection, attentional control, and decision-making (as somatosensory cortex, anterior cingulate cortex, amygdala, anterior insula, and prefrontal cortex) are involved in pain processing (Lamm, Decety, & Singer, 2011). Interestingly, recent studies demonstrated that these same systems are also involved in the handling of social situations in which some level of suffering (yet no actual physical pain) is occurring (Eisenberger, 2012). This is observed during the visualization of images of painful situations, where the neural underpinnings of empathy for the pain of others can also be observed (Masten, Morelli, & Eisenberger, 2011). Which brain areas and processes are shared by physical pain and social pain is a topic of great relevance in the field today, and some studies using tDCS have helped to elucidate that.
In one of the first studies to investigate pain processing using tDCS, Boggio, Zaghi, Lopes, and Fregni (2008) started testing the role of the primary motor cortex (M1), the left primary visual area (V1), and left dorsolateral prefrontal cortex (left DLPFC) in pain processing. The volunteers received anodal tDCS over these structures in separate sessions and received electrical peripheral stimulation (painful and not painful) on their hands. They only found significant results for anodal stimulation over M1 and DLPFC. M1 tDCS increased both somatosensory and pain thresholds, while DLPFC tDCS only increased pain thresholds. The authors discussed these results, highlighting the important influence of both structures (DLPFC and M1) on pain processing and emphasizing a differential role of these structures in pain processing. In this experiment, pain was induced by electrical stimulation. One important debate is about pain induced by observing others in pain.
To investigate this issue, the same group conducted a similar study in which the same tDCS conditions were applied, but participants had to judge the unpleasantness of pictures presenting human beings under painful conditions (Boggio, Zaghi, & Fregni, 2009). The results showed a significant decrease in unpleasantness and discomfort assessment during anodal left DLPFC tDCS in relation to sham tDCS, while no other significant effects were found on other conditions. These findings demonstrate that DLPFC is a critical area for the emotional processing of pain. As M1 tDCS did not modulate unpleasantness and discomfort assessment, authors also suggested different pathways for emotional pain and somatosensory perception. In a similar task as Boggio et al. (2009) but with positive and neutral human pictures added, Peña-Gómez, Vidal-Piñeiro, Clemente, Pascual-Leone, and Bartrés-Faz (2011) found no significant effects for valence assessment of positive and neutral pictures under left DLPFC anodal tDCS in addition to confirming previous findings. Their findings highlight the possible involvement of this structure in the specific processing of negative content and the subsequent emotional regulation of such content.
In a similar study, Feeser, Prehn, Kazzer, Mungee, and Bajbouj (2014) investigated the role of the DLPFC in an emotional regulation task (up-regulating and down-regulating the current negative emotion). Anodal tDCS was applied to the right DLPFC with the cathodal electrode over the supraorbital contralateral region. The skin conductance response (SCR) was also recorded. They demonstrated that when up-regulating negative emotions, participants who underwent active tDCS had higher SCR levels and arousal ratings than participants who received sham tDCS. On the other hand, during the down-regulation of negative emotions, smaller SCR levels followed by lower arousal assessments were observed for active compared to sham tDCS. These findings suggest that increased activity in the right DLPFC could be linked to increased cognitive control on emotion regulation (Ochsner, Silvers, & Buhle, 2012).
Interestingly, not just pain can be modulated via tDCS. Bolognini, Miniussi, Gallo, and Vallar (2013) demonstrated that tDCS is able to modulate touch synesthesia. Bolognini et al. (2013) found that anodal tDCS of the left or right somatosensory cortex induced the manifestation of synesthesia-like effects (being less accurate and slower to identify touch in self when observing incongruent touch in others) in non-synesthetic participants. Additionally, they found a positive correlation between perspective-taking score (a subscale of the Interpersonal Reactivity Index) and the synesthesia-like effect induced by tDCS.
The above-mentioned studies show that tDCS can be used to study the neural mechanisms behind understanding others’ somatic sensations, pain perception, judgment of painful situations, and emotion regulation.
Another different phenomenon, generally known as social pain, can be characterized as the experience of suffering due to personal losses or rejection and ostracism (Eisenberger, 2012; Lieberman & Eisenberger, 2006; Van Beest, Williams, & Van Dijk, 2011; Williams, 2007). Typically, it is observed that under these conditions, there is a decrease in mood and basic needs levels (belonging, self-esteem, control, and meaning of existence). A recent study by Kelley, Hortensius, and Harmon-Jones (2013) showed that when submitted to right DLPFC anodal tDCS, participants presented higher levels of rumination while being ostracized in the so-called Cyberball (see Williams & Jarvis, 2006 for review). This effect points to the role of the right DLPFC and, particularly, the effects of an imbalance in the interhemispheric activity. It is interesting to observe that rumination is a very common behavior in patients with major depression and that it is accompanied by increased right DLPFC activity and decreased activity of the contralateral homologous structure (Coan & Allen, 2004).
Another brain area stimulated by tDCS during ostracism induced by the Cyberball is the ventrolateral prefrontal cortex (VLPFC; Riva, Lauro, DeWall, & Bushman, 2012), which showed that anodal tDCS over the right VLPFC could reduce the discomfort and feelings of pain compared to sham tDCS. More recently, the same group showed that under the same protocol, participants who received active tDCS reported lower levels of aggressiveness after the ostracism task (Riva, Lauro, DeWall, Chester, & Bushman, 2014). A similar effect in aggressive behavior was also achieved with anodal tDCS stimulation over the right DLPFC, which led to diminished levels of self-reported aggressiveness in men (Dambacher et al., 2015).
Altogether, these studies provide causal evidence of the role of the prefrontal cortex in emotional control processes and emotion reappraisal (Ochsner et al., 2012). These studies highlight the relevance of tDCS to the study of pain, empathy for pain (see Hétu, Taschereau-Dumouchel, & Jackson, 2012, for a discussion of this issue), and social pain phenomena.
Social Interaction
The processing of social information that supports social interaction is a central topic in social neuroscience and psychology. Among the many processes and abilities subserving social interaction, we highlight three in which tDCS research has provided relevant insights: perception of facial expression, perspective taking, and imitation. Here, we present the main studies using tDCS in the investigation of these abilities.
Most of the research using neuromodulation to investigate facial expression perception has focused on elucidating the brain networks involved in emotion detection. A seminal study on this topic used TMS to inhibit the medial prefrontal cortex (Harmer, Thilo, Rothwell, & Goodwin, 2001) while participants viewed faces with angry or happy expressions. This study found that disruption of the medial prefrontal cortex affected only angry face perception. Since that study, others have been conducted supporting the existence of different networks to process specific emotions (e.g., Ferrucci et al., 2012; Nitsche et al., 2012) and the existence of brain processing differences between male and female volunteers in specific emotion perception (Boggio, Rocha, da Silva, & Fregni, 2008).
The first study to use tDCS in the investigation of emotional face processing evaluated males and females playing a face expression go-no-go task (Boggio, Rocha, et al., 2008). In this task, photographs of sad, happy, and neutral faces were shown to the participants, and in each block, either sad or happy was specified as the target. The participants were submitted to bilateral tDCS stimulation over the temporal cortex, with the anodal electrode over left and cathodal electrode over the right temporal cortex. The results showed that women made fewer errors with active stimulation compared to the sham when sad faces were the targets. Contrarily, men made more errors in the same condition (sad faces as target) with active stimulation compared to the sham. A possible hypothesis raised by the authors is the existence of different networks subserving sad face perception in women and men.
Other studies investigated the involvement of specific brain areas in the processing of facial expressions. The study by Ferrucci et al. (2012) investigated the involvement of the cerebellum and right prefrontal cortex in the perception of negative, positive, and neutral facial expressions. They found that only cerebellar stimulation (for both anodal and cathodal) enhanced the perception of negative (anger and sadness) facial expressions when compared to sham stimulation. Another study by Nitsche et al. (2012) evaluated the involvement of the left and DLPFC cortex in emotional state and emotional face identification. The participants were submitted to tDCS stimulation over the left DLPFC, while the reference electrode was placed over the right frontopolar cortex. The authors found that anodal stimulation over the left DLPFC led to improved positive emotional face recognition.
These experiments provide important information about brain structures and their roles in the perception of emotional faces. Nevertheless, the use of tDCS to understand face perception is still at its beginning. New studies are necessary to obtain a deeper comprehension of specific brain structures as well as the neural circuitries underlying face perception. Additionally, new tDCS experiments might help to clarify possible differences between genders with regard to face processing. The construction of affective, social, and cognitive models based on the causal effects promoted by tDCS might help in the investigation of social deficits typically observed in some clinical populations such as autism. In particular, the integration of tDCS with other techniques such as eye tracking systems will possibly answer important questions about the static and dynamic face tracking abnormalities observed in autism and other conditions.
As mentioned previously, social interaction also depends on perspective taking, an essential ability related to empathy and consequently to the development and maintenance of positive social connections (Seyfarth & Cheney, 2013). A relevant study investigated the neuromodulation of temporoparietal junction (TPJ) in participants’ performance on three social cognition tasks: on a motor imitation task, a spatial perspective-taking task, and a self-referential task. Although neuroimaging studies have shown the involvement of TPJ in abilities related to the execution of these tasks, TPJ tDCS effects were not the same for all tasks. This study showed that anodal TPJ tDCS improved the control of self-other discrimination related to the imitation and perspective-taking tasks, while it did not have any effect on mental attribution ability, as evaluated by the self-referential task (Santiesteban, Banissy, Catmur, & Bird, 2012). This study helped to clarify the involvement of TPJ in empathy, confirming its main role in the specific function of self-other discrimination. Hogeveen et al. (2014) expanded these findings by testing the effects of anodal tDCS over the right TPJ or right inferior frontal cortex (IFC) on imitative control functions. Interestingly, anodal tDCS of the right IFC improved the ability to inhibit imitation in a task in which it was required but, at the same time, increased the imitation during a social interaction task (which is related to better social interaction). Thus, it seems that IFC is related to imitation control depending on the performing task. With regard to anodal tDCS over TPJ, a positive effect was observed in the ability to inhibit imitation but had no effect on the imitation during the social interaction task. These findings support the notion of a direct role of the IFC in imitative behavior and an indirect role of the TPJ.
The possibility of promoting imitative behavior by brain stimulation opens an avenue of investigations on its use as a tool to promote social plasticity. New studies of tDCS on social abilities might point to the future possible clinical use in individuals with developmental disorders that present social cognition impairments such as autism or schizophrenia.
Prejudice
The frequency of sexual, social, or racial prejudice in human interactions is significant. Although the frequency of explicit demonstrations of prejudice seems to (arguably) be diminishing in most cultures, implicit prejudice appears to be present in many circumstances and remains a very relevant topic in contemporary neuroscience research (see Kubota, Banaji, & Phelps, 2012, for a discussion of this issue in racial prejudice studies). Among all research tools in this field, one that is used most is the Implicit Association Test (IAT). It allows for the investigation of interactions between different stimulus categories (e.g., Caucasian and African-American faces and positive and negative valence words) in a fast forced-choice task that unveils biased associations that are frequently not explicitly accessible (Greenwald, McGhee, & Schwartz, 1998).
More recently, some groups investigated prejudice and its implicit associations using neuromodulation techniques such as TMS and tDCS. TMS studies showed that inhibiting the left DLPFC function was able to increase the participant’s gender bias (Cattaneo, Mattavelli, Platania, & Papagno, 2011) and religiousness-spirituality bias (Crescentini, Aglioti, Fabbro, & Urgesi, 2014) during IAT. These findings suggest that increased activity in the left DLPFC might be related to decreased inhibitory control, unveiling stereotyped responses.
A recent tDCS work has also investigated the role of the left DLPFC in mediating implicit bias in responses to nonsocial stimuli, finding interesting results that are somewhat complementary to those mentioned above. Using an IAT task, Gladwin, den Uyl, and Wiers (2012) found that tDCS of the left DLPFC did not affect the implicit bias processes in the association of insect images and insect names. Taken together with the works of Cattaneo et al. (2011) and Crescentini et al. (2014), these results could be interpreted to suggest that there is something special in the left DLPFC concerning the processing of social (in contrast to nonsocial) bias. In fact, Cattaneo et al. (2011) reviewed evidence from fMRI studies supporting this hypothesis.
The studies mentioned in this brief section show that tDCS and TMS can be effective tools in the investigation of the underlying mechanisms of prejudice and implicit social biases. Nonetheless, there are very few investigations of these subjects that employ such techniques. Considering the social burden that is associated with prejudice in our society today, more studies on prejudice employing neuromodulatory techniques are recommended.
Social Decision-Making
In the fields of economics and psychology, social decision-making is a topic that investigates how a person chooses between alternatives in the context of social interaction (Sanfey, 2007). In the last decade, methods and techniques from neuroscience have been applied to investigate the neurobiological substrates of social decision-making. Despite the fact that most studies combining social decision-making and neuroscience focused on neuroimaging methods, some relevant studies used neuromodulation techniques and handled useful information about the role of different brain areas in decision-making and how controlled and automatic processes interact in the decision-making processes (Loewenstein, Rick, & Cohen, 2008).
A relevant research topic in social decision-making is the investigation of the neurobiological underpinnings of fairness perception and compliance with social norms. A pioneer study in this topic was conducted by Knoch et al. (2006). They used low-frequency TMS to inhibit the right DLPFC activity while participants played the Ultimatum Game (UG). The UG is a resource-sharing task used to investigate the reaction to unfairness. In this game, two participants are given an initial asset and one of them first proposes a sharing rate and the other participant accepts it (both of them gain the proposed value) or rejects it (both gain nothing). Neuroimaging studies showed increased activity of the anterior insula and right DLPFC in participants facing the unfair proposals. However, it was not clear whether the activity of the DLPFC, an area related to cognitive control, including the top-down control of automatic and prevalent responses, was related to the inhibition of an impulse to reject unfairness or, contrarily, to seek gains independently of quantity. The manipulation of the right DLPFC through TMS showed that the inhibition of this area led to a higher acceptance of unfair proposals, confirming the hypothesis that human beings have an impulse to approach gains and that this temptation would be controlled by the activity of areas related to high-level cognitive control.
The seminal study by Knoch et al. (2006) was followed by a study by Knoch and Fehr (2007) in which they applied inhibitory tDCS over the right DLPFC during a UG task. They found similar results using tDCS compared to their previous TMS study (Knoch et al., 2006): enhanced acceptance of unfair proposals due to the inhibition of the right DLPFC activity. Given these similar results, Knoch and Fehr (2007) considered the potential use of tDCS relative to TMS in tasks with simultaneous social interaction, given the previously presented advantages of tDCS.
The regular UG task assesses the effect of unfairness on respondents as the proposal’s recipients. Recent experiments have begun to investigate the effects of unfairness when the responder must decide for him/herself (myself condition) or on behalf of a third-party (third-party condition) (Civai, Crescentini, Rustichini, & Rumiati, 2012). Interestingly, inequity aversion was observed in both the myself and third-party conditions. Nevertheless, the MPFC was strongly activated during the myself condition. To test the causal role of the MPFC in personal damage versus general inequity aversion, Civai, Miniussi, and Rumiati (2014) investigated the effect of cathodal tDCS over the MPFC in this modified version of the UG. They found that cathodal tDCS over MPFC led to diminished rejection of unfair proposals in the myself condition. In agreement with previous fMRI experiments, these findings provide evidence that MPFC is related to fairness processing when the self is involved.
Another study using tDCS to modulate social decision-making was conducted by Ruff, Ugazio, and Fehr (2013). They used a task similar to the UG first used by Spitzer, Fischbacher, Herrnberger, Grön, and Fehr (2007). In this game, two players divide an initial endowment. One player is a proposer and suggests a division rate to a second player, the receiver. The experimenters created two different conditions for this game: a control and a punish condition. In the control condition, the receiver could only accept the proposal passively, similar to a dictator game. In the punish condition, the receiver could spend some money to punish the proposer. After the initial endowment, the players received extra money (used by the receivers to punish the proposer in the punishment condition). A neuroimaging study by Spitzer et al. (2007), the first using this task, found that the punishment condition led the proposers to comply with the social norms and share the endowment more fairly, and this behavioral adaptation was related to an enhanced activation of the right DLPFC, left ventrolateral prefrontal cortex, and bilateral orbitofrontal cortex. Ruff et al. (2013) modulated the right DLPFC with anodal and cathodal stimulation to investigate the right DLPFC role on norm compliance. They found that in the punishment condition, the anodal stimulation (compared to sham) led the proposer to transfer more money after punishment, enhancing the norm compliance. In contrast, the cathodal stimulation made the proposers more self-interested and less oriented by social norms of fairness, diminishing the quantity transferred to the receivers. In the control condition (where the receiver could only accept passively), the stimulation acted in a contrary way. During anodal stimulation, proposers shared less with the receiver, while the cathodal stimulation led to fairer sharing. While they found behavioral changes due to the neuromodulation, they did not find any changes in the perception or expectation of social norms. This result suggests that the right DLPFC is involved in a network linked to norm compliance. However, it is not clear why neuromodulation of the right DLPFC acted in contrary ways between the punish and control conditions. A hypothesis raised by Sanfey, Stallen, and Chang (2014) to explain these data suggested that given the role of the right DLPFC in expectation processing as presented in previous studies, the expected norms in the punish and control conditions could be different, with participants expecting that people would offer less money in the control condition and more money in the punishment condition. Nevertheless, the norm expectations for each condition were not evaluated and remain an open question. A suggestion by Sanfey et al. (2014) was that the right DLPFC worked together with other areas such as the anterior cingulate cortex and insula in a network related to compliance. While these two latter areas would be related to a failure in expectancy, the right DLPFC may be related to goal maintenance and the cognitive control to achieve that goal.
The above-mentioned studies provide many possibilities for the clinical use of tDCS in neurological or psychiatric disorders in which compliance with social norms is defective (Ruff et al., 2013). Nonetheless, tDCS-aided interventions for social cognition rehabilitation are still in their infancy.
Conclusion and Future Directions
The present review argues that our understanding of social neuroscience and psychology would benefit from more research using noninvasive brain stimulation methods. More importantly, we argue that tDCS is an effective, safe, and low-cost tool for that purpose. In fact, the present review shows a number of papers that have used tDCS in investigations that have advanced our knowledge of the brain substrate involved in social pain and empathy for pain, implicit associations involved in prejudice-related behavior, social interaction, and social decision-making. Here, we argue that although tDCS is still in its infancy as a tool for social neuroscience and psychology studies, we have passed the point in which researchers should be asking “if” or “how” tDCS might be used for social neuroscience research. We believe that we are now at the point where many researchers are asking “why not more?” or how to do better, and we are certainly among them. Figure 3 (showing the number of publications per year that mentioned the terms “Transcranial Direct Current Stimulation” and “Social”) supports that this is a growing trend, although the use of tDCS in the field is still much less popular than TMS (see Figure 1).
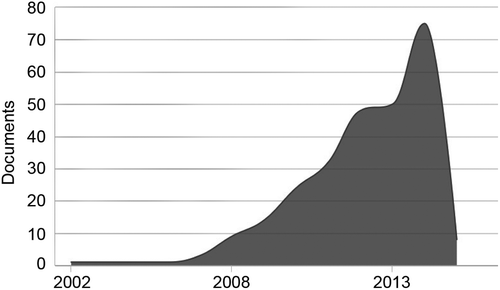
Regarding future directions, we highlight three main issues in the intersection between Social Neuroscience and tDCS research that are critical for the advancement of the field. First, more studies are needed. As we argued before, this technique needs to be used more frequently in this field. Second, more replication studies are needed, as in every new field. Lastly, more studies with clinical populations should be done. As there is growing evidence for the tDCS potential in neurorehabilitation (Brunoni et al., 2012; Brunoni, Valiengo, et al., 2013) and there are many conditions that strongly impact social cognition with limited choices of treatment (e.g., dementias, traumatic brain injuries, autism spectrum disorder, schizophrenia), it is critical that more investigations are done on the clinical potential of this technique in social rehabilitation. There is a whole avenue of research still to be explored and the promising results showing tDCS can be effective in the treatment of depression (Brunoni, Boggio, et al., 2013) and pain (Mori et al., 2010) justify these investigations.
PSB is a CNPq research fellow and is supported by National Council for Scientific and Technological Development (CNPq – 480891/2012-5). The authors declare no competing financial interests.
Paulo S. Boggio is a Senior Researcher and Professor of Neuroscience and Behavior, Affiliate Member of the Brazilian Academy of Sciences, CNPq Research Fellow, and Director of the Social and Cognitive Neuroscience Laboratory at the Mackenzie Presbyterian University, Brazil. His research interests include Neuromodulation and Social Cognition.

Gabriel G. Rêgo is a PhD Student at the Social and Cognitive Neuroscience Laboratory at the Mackenzie Presbyterian University, Brazil. His major research interests include Decision-Making, Neuroeconomy, Social Psychology, and Neuromodulation.

Lucas M. Marques is a Master Student at the Social and Cognitive Neuroscience Laboratory at the Mackenzie Presbyterian University, Brazil. His major research interests include Emotion, Emotion Regulation, Psychophysics, and Neuromodulation.

Thiago L. Costa is a Postdoctoral Research at the Social and Cognitive Neuroscience Laboratory at the Mackenzie Presbyterian University, Brazil. His research mostly focuses on perception, spanning from low-level visual processing and perceptual organization to higher order cognitive processes.

References
(2005). Side effects of transcranial magnetic stimulation biased task performance in a cognitive neuroscience study. Brain Topography, 17, 193–196.
(2004). Neural signals for the detection of unintentional race bias. Psychological Science, 15, 88–93.
(2009). Decreasing procedural pain over time of left prefrontal rtms for depression: Initial results from the open-label phase of a multisite trial (OPT-TMS). Brain Stimulation, 2, 88–92.
(2004). The role of emotion in decision-making: Evidence from neurological patients with orbitofrontal damage. Brain and Cognition, 55, 30–40.
(2008). Differential modulatory effects of transcranial direct current stimulation on a facial expression go-no-go task in males and females. Neuroscience Letters, 447, 101–105.
(2009). Modulation of emotions associated with images of human pain using anodal transcranial direct current stimulation (tDCS). Neuropsychologia, 47, 212–217.
(2008). Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. European Journal of Neurology, 15, 1124–1130.
(2013). Induction of mirror-touch synaesthesia by increasing somatosensory cortical excitability. Current Biology, 23, R436–R437.
(2014). The neuroethology of friendship. Annals of the New York Academy of Sciences, 1316, 1–17.
(2011). A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. International Journal of Neuropsychopharmacology, 14, 1133–1145.
(2013). Transcranial direct current stimulation: Challenges, opportunities, and impact on psychiatry and neurorehabilitation. Frontiers in Psychiatry, 4, 1–3. doi: 10.3389/fpsyt.2013.00019
(2012). Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimulation, 5, 175–195.
(2013). The sertraline vs. electrical current therapy for treating depression clinical study: Results from a factorial, randomized, controlled trial. JAMA Psychiatry, 70, 383–391.
(1992). Social psychological contributions to the decade of the brain: Doctrine of multilevel analysis. The American Psychologist, 47, 1019
(2011). The role of the prefrontal cortex in controlling gender-stereotypical associations: A TMS investigation. NeuroImage, 56, 1839–1846.
(2012). Equality versus self-interest in the brain: Differential roles of anterior insula and medial prefrontal cortex. NeuroImage, 62, 102–112.
(2014). Medial prefrontal cortex reacts to unfairness if this damages the self: A tDCS study. Social Cognitive and Affective Neuroscience, 10, 1054–1060.
(2004). Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology, 67, 7–50.
(2014). Virtual lesions of the inferior parietal cortex induce fast changes of implicit religiousness/spirituality. Cortex, 54, 1–15.
(1994). The return of Phineas Gage: Clues about the brain from the skull of a famous patient. Science, 264, 1102–1105.
(2015). Reducing proactive aggression through non-invasive brain stimulation. Social Cognitive and Affective Neuroscience, 10, 1303–1309. doi: 10.1093/scan/nsv018
(2003). Social modulation of stress responses. Physiology & Behavior, 79, 399–407.
(2013). Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: A basis for high-definition tDCS. NeuroImage, 74, 266–275.
(2012). The pain of social disconnection: Examining the shared neural underpinnings of physical and social pain. Nature Reviews: Neuroscience, 13, 421–434.
(2014). Transcranial direct current stimulation enhances cognitive control during emotion regulation. Brain Stimulation, 7, 105–112.
(2012). Cerebellum and processing of negative facial emotions: Cerebellar transcranial DC stimulation specifically enhances the emotional recognition of facial anger and sadness. Cognition & Emotion, 26, 786–799.
(2008). Cerebellar transcranial direct current stimulation impairs the practice-dependent proficiency increase in working memory. Journal of Cognitive Neuroscience, 20, 1687–1697.
(2014). Applications of transcranial direct current stimulation for understanding brain function. Trends in Neurosciences, 37, 742–753.
(2008). Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: A randomized, sham-controlled study. Journal of Clinical Psychiatry, 69, 32–40.
(2012). Anodal tDCS of dorsolateral prefontal cortex during an Implicit Association Test. Neuroscience Letters, 517, 82–86.
(1998). Measuring individual differences in implicit cognition: The implicit association test. Journal of Personality and Social Psychology, 74, 1464.
(2001). Transcranial magnetic stimulation of medial-frontal cortex impairs the processing of angry facial expressions. Nature Neuroscience, 4, 17–18.
(2012). Stimulating the brain to study social interactions and empathy. Brain Stimulation, 5, 95–102.
(2014). Inability to empathize: Brain lesions that disrupt sharing and understanding another’s emotions. Brain, 137, 981–997.
(2014). Task-dependent and distinct roles of the temporoparietal junction and inferior frontal cortex in the control of imitation. Social Cognitive and Affective Neuroscience, 10, 1003–1009.
(2014). Identifying autism from neural representations of social interactions: Neurocognitive markers of autism. PLoS One, 9, e113879.
(2013). When anger leads to rumination induction of relative right frontal cortical activity with transcranial direct current stimulation increases anger-related rumination. Psychological Science, 24, 475–481.
(2007). Resisting the power of temptations. Annals of the New York Academy of Sciences, 1104, 123–134.
(2006). Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. The Journal of Neuroscience, 26, 6469–6472.
(2008). Studying the neurobiology of social interaction with transcranial direct current stimulation – the example of punishing unfairness. Cerebral Cortex, 18, 1987–1990.
(2012). The neuroscience of race. Nature Neuroscience, 15, 940–948.
(2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage, 54, 2492–2502.
(2006). A pain by any other name (rejection, exclusion, ostracism) still hurts the same: The role of dorsal anterior cingulate cortex in social and physical pain. Social Neuroscience: People Thinking About Thinking People, 1, 169–187.
(2007). Social cognitive neuroscience: A review of core processes. Annual Review of Psychology, 58, 259–289.
(2008). Neuroeconomics. Annual Review of Psychology, 59, 647–672.
(2008). A review of the safety of repetitive transcranial magnetic stimulation as a clinical treatment for depression. International Journal of Neuropsychopharmacology, 11, 131–147.
(2008). Phineas Gage-unravelling the myth. The Psychologist, 21, 828–831.
(2011). An fMRI investigation of empathy for “social pain” and subsequent prosocial behavior. NeuroImage, 55, 381–388.
(2012). Neurobiological effects of transcranial direct current stimulation: A review. Frontiers in Psychiatry, 3, 1–11. doi: 10.3389/fpsyt.2012.00110
(2013). Modelling non-invasive brain stimulation in cognitive neuroscience. Neuroscience and Biobehavioral Reviews, 37, 1702–1712.
(2010). Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis. The Journal of Pain, 11, 436–442.
(2008). Transcranial direct current stimulation: State of the art 2008. Brain Stimulation, 1, 206–223.
(2012). Effects of frontal transcranial direct current stimulation on emotional state and processing in healthy humans. Frontiers in Psychiatry, 3, 1–10.
(2004). GABAergic modulation of DC stimulation-induced motor cortex excitability shifts in humans. European Journal of Neuroscience, 19, 2720–2726.
(2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1–E24.
(2000). Transcranial magnetic stimulation in cognitive neuroscience-virtual lesion, chronometry, and functional connectivity. Current Opinion in Neurobiology, 10, 232–237.
(2011). Down-regulation of negative emotional processing by transcranial direct current stimulation: Effects of personality characteristics. PLoS One, 6, e22812.
(2012). Buffer the pain away stimulating the right ventrolateral prefrontal cortex reduces pain following social exclusion. Psychological Science, 23, 1473–1475.
(2014). Reducing aggressive responses to social exclusion using transcranial Direct Current Stimulation (tDCS). Social Cognitive and Affective Neuroscience, 10, 352–356.
(2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120, 2008–2039.
(2013). Changing social norm compliance with noninvasive brain stimulation. Science, 342, 482–484.
(2007). Social decision-making: Insights from game theory and neuroscience. Science, 318, 598–602.
(2014). Norms and expectations in social decision-making. Trends in Cognitive Sciences, 18, 172–174.
(2012). Enhancing social ability by stimulating right temporoparietal junction. Current Biology, 22, 2274–2277.
(2013). Affiliation, empathy, and the origins of theory of mind. Proceedings of the National Academy of Sciences, 110, 10349–10356.
(2007). The neural signature of social norm compliance. Neuron, 56, 185–196.
(2009). Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. The Journal of Neuroscience, 29, 5202–5206.
(2011). Physiological basis of transcranial direct current stimulation. The Neuroscientist, 17, 37–53.
(2011). Cyberbomb Effects of being ostracized from a death game. Group Processes & Intergroup Relations, 14, 581–596.
(2014). Investigation of tDCS volume conduction effects in a highly realistic head model. Journal of Neural Engineering, 11, 016002.
(2007). Ostracism. Annual Review of Psychology, 58, 425–452.
(2006). Cyberball: A program for use in research on interpersonal ostracism and acceptance. Behavior Research Methods, 38, 174–180.
(2010). Disruption of the right temporoparietal junction with transcranial magnetic stimulation reduces the role of beliefs in moral judgments. Proceedings of the National Academy of Sciences, 107, 6753–6758.
(1969). Relative effects of prolonged social isolation and confinement: Behavioral and EEG changes. Journal of Abnormal Psychology, 74, 625.



