Systematic Review of Sensory Stimulation Programs in the Rehabilitation of Acquired Brain Injury
Abstract
Abstract. Acquired Brain Injury (ABI) can lead to sensory deficits and compromise functionality. However, most studies have been focused on motor stimulation in stroke and traumatic brain injury (TBI). Sensory stimulation in stroke and mild/moderate TBI has received reduced interest. The main objective of this review is to know the methodological characteristics and effects of sensory programs in ABI. Studies with the purpose of testing the efficacy of those programs were identified through a literature search, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Cochrane Collaboration Guidelines. Twenty-three studies were included in this review. The results show that in most studies sensory stimulation started within 12 months after injury and there is no consensus regarding frequency, duration and number of sessions, duration of intervention, and instruments used to assess outcomes. Most programs involved unisensory stimulation, and vision was the predominant target. The most used methods were compensation and somatosensory discrimination training. Most studies used a pre- and post-intervention assessment, with few studies comprising follow-up assessment. Regarding the studies revised, the interventions with positive outcomes in ABI are: compensation, cognitive training, vestibular intervention, somatosensory discrimination training, proprioceptive stimulation with muscle vibration, and sustained attention training with olfactory stimulation. Available findings suggest that sensory stimulation has positive results with immediate and long-term improvements in sensory functioning. This review provides useful information to improve rehabilitation and to design future investigation.
Acquired Brain Injury (ABI) is defined as brain damage not related to a congenital defect nor degenerative disease that may cause temporary or permanent dysfunctions and poor psychosocial adjustment (World Health Organization [WHO], 1996). The most prevalent causes for ABI worldwide are traumatic brain injury (TBI) and stroke, which in turn are relevant causes of death and incapacity (Feigin et al., 2017; Hawryluk & Bullock, 2016). Depending on the nature and location of the lesion, sensory, cognitive, motor, behavioral, and emotional deficits can emerge. These alterations may have impacts in activities of daily living, social participation, and occupational re-integration. Therefore, rehabilitation should be initiated as soon as possible, targeting motor, sensory, cognitive, and affective functioning. However, the clinical heterogeneity of the ABI groups and of the interventions used, pose a serious challenge to the research and intervention in this field (Turner-Strokes, 2003).
About 60% of the individuals present sensory deficits after ABI (Carey, 1995). ABI severity is positively correlated with the severity of the sensory compromise (Tyson et al., 2008). Connell (2007) points that 46–71% of the variance regarding recuperation is explained by the severity of the ABI, sensory commitment, and daily living functionality, suggesting that cognitive factors and perceptive ability affect this result.
TBI may lead to damage of sensory receptors and sensory pathways (Folmer et al., 2011). Despite the higher prevalence of mild and moderate TBI in comparison to severe TBI (Li et al., 2016), there is a lesser interest in the sensory stimulation in the two first forms. Padilla and Domina (2016) found evidence that multimodal stimulation improves arousal and clinical outcomes of comatose patients or in persistent vegetative state. As a result, these authors recommend the early implementation of sensory stimulation, with intensive frequency (3–5 times/day, 20 min sessions) and increasing complexity of the programs, adjusting the stimuli to patient’s pre-morbid preferences.
Sensory stimulation consists in the controlled exposition to specific sensory stimuli or environments through different procedures (Padilla & Domina, 2016). Most of the intervention protocols for persons with consciousness disorders encompass simple, frequent, and repetitive stimuli, which may have emotional or autobiographical content. The use of natural or virtual environments with complex stimuli is considered as being more effective than artificial environments, as the former require cognitive processing of input and output information and allow the introduction of emotional and autobiographical contents (Abbate et al., 2014).
The stimulation strategies can be directed to one sense only (unimodal or unisensory stimulation) or several senses (multimodal or multisensory stimulation). Senses can be divided in primary modalities, such as touch, pressure, pain, temperature, position, and vibration, and secondary modalities, such as tactile discrimination, stereognosis, graphesthesia, tactile localization, among others. Regarding vision and hearing, a lesion in the primary visual cortex or in the primary auditory cortex often originates sensory-perceptive disturbances, and lesions in the association cortices may cause incapacity to recognize and interpret stimuli (Campbell, 2013).
Concerning visual rehabilitation, three methods are usually considered: (a) adaptation or compensation; (b) substitution; and (c) restoration (Zangwill, 1947). Compensation aims to adapt patients to the loss of visual field, namely through the training of visual search strategies. Substitution strategies aim to change visual field using apparatus such as prisms, eye-patches, and magnifying glasses (Rowe et al., 2013). Restoration is mostly achieved by the stimulation of the neural areas in the transition between the compromised and intact visual fields (Bergsma et al., 2012).
Hemineglect is one of the common sequelae of both stroke and TBI. The treatment approaches can be divided into extrinsic or top-down, intrinsic or bottom-up, and combined. The top-down approaches make use of external orientations in order to elicit active, conscious, and meaningful participation of the patient; bottom-up approaches do not require patients’ active participation, once these approaches are based on the manipulation the characteristics of the stimuli or direct activation of the brain itself. Top-down approaches include training of visual scanning, sustained attention, and mental imagery, while bottom-up include prism glasses, phasic alert, torso rotation, eye-patches, limb activation, optokinetic and thermal stimulation, transcutaneous electric stimulation, neck muscles vibration, and direct cerebral stimulation. Combined strategies are also possible, for example including prism adaptation and visual search training (Marshall, 2009).
The need for sensory intervention has been justified with several factors. Sensory deficits compromise the ability to explore the environment (Carey et al., 1993), the functionality in activities of daily living (Nelles et al., 2010), are related to a reduction in social participation (Carey et al., 2018; Rowe et al., 2013), and associated with cognitive deficits (Schubert et al., 2017). For example, not only visual and auditory deficits have a negative impact on cognitive functioning, but reduction in processing speed and executive deficits also have a negative influence on visual and auditory processing (Lew et al., 2010). Thus, authors like Sullivan and Hedman (2008) point to the need of further studies, namely to determine methodological issues such as the dose-response ratio in sensory stimulation in order to better adjust the rehabilitation programs.
Summing-up, previous systematic reviews assessed the effects of: (a) multisensory stimulation after ABI on low- and higher-level sensory deficits (Tinga et al., 2016); (b) multimodal stimulation in persistent vegetative state or comatose patients (Padilla & Domina, 2016); (c) different therapies for hemineglect after ABI (Bowen et al., 2013; Kashiwagi et al., 2018; Lisa et al., 2013; Yang et al., 2013); (d) sensory stimulation on motor recuperation (Grant et al., 2018; Laufer & Elboim-Gabyzon, 2011).
To our knowledge this is the first systematic review aiming to analyze the methodological characteristics of sensory stimulation programs for the rehabilitations of ABI patients. The present work also intended to systematically review the effects of sensory stimulation on cognitive rehabilitation and to determine the most frequent clinical conditions in which they are used. Regarding the impact of the sensory deficits the review focused on programs directed to the stimulation of primary and secondary sensory modalities. The description of these programs could be useful for the identification of the dose-response ratio in order to adjust the clinical practice and give directions to future research. The following research questions were formulated:
How long after the ABI the sensory stimulation is usually initiated?
What are the most frequently used methods in sensory stimulation programs and what are the most simulated senses?
What is the usual frequency, duration, number of sessions, and length of sensory stimulation programs?
Regarding intervention outcomes, research questions were the following:
Which assessment instruments are most frequently used to evaluate the efficacy of sensory stimulation?
What are the immediate and long-term outcomes of the sensory stimulation programs?
Are the outcomes of the stimulation programs generalizable to cognitive functioning or activities of daily living?
Method
The protocol of the systematic review followed the recommendations of the Preferred Systematic Review and Meta-analysis (PRISMA; Moher et al., 2009; Shamseer et al., 2015).
Search Strategy
Studies were identified through a search on EBSCO, PubMed and OTseeker. The following EBSCO databases were selected: MEDLINE, MEDLINE with full text, Academic Search Complete, PsycInfo, PsycArticles, CINAHL Plus with full text and Psychology and Behavioral Sciences Collection. Furthermore, references of the selected studies were reviewed in order to identify other relevant studies. A manual search was performed to prevent source selection bias.
The search strategy comprised three types of terms related to the population, sensory component and intervention. The search equation was: brain injur* OR brain damag* OR tbi OR head trauma OR cerebrovascular disorders OR cerebrovascular accident* OR cva OR stroke OR poststroke AND multisensor* OR sensor* OR multimodal OR visual* OR tactile* OR vestibular OR audio* OR olfactory OR Somatosens* OR percep* OR visuo* OR audit* OR neglect OR propriocep* AND rehabilitation OR remediation OR education OR *training OR treatment* OR therapy OR stimulation OR integration. All expressions were limited to the titles.
Studies Selection
Inclusion criteria were: (a) adult participants (+18 years) with ABI; (b) empirical studies aiming to assess the methodology used in sensory stimulation programs; (c) human studies only; (d) publications in English language only. The following constituted exclusion criteria: (a) literature reviews; (b) studies with only motor stimulation or presenting only motor outcomes; (c) studies with no relevance for the theme; (d) studies using only cerebral stimulation techniques; (e) case studies; (f) studies including only participants in coma or in vegetative persistent state.
After the elimination of duplicate articles, the selection of studies for full-test analysis was performed by two independent reviewers in accordance with the Cochrane Collaboration’s recommendations (Higgins & Green, 2011). A third reviewer was consulted to decide dissent cases in article selection. After full-text analysis, articles previous to 1990 were also eliminated due to methodological limitations, such as reduced number of participants, unclear intervention description, or lack of outcomes of the intervention.
Results
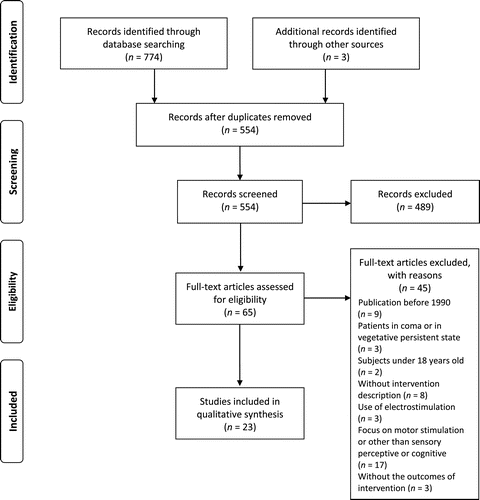
A total of 774 papers were identified (EBSCO: n = 278, PubMed: n = 436, OTseeker: n = 60). Three papers were included through manual search. After the exclusion of duplicate papers, the analysis of titles and abstracts of 554 papers was carried by two researchers. Sixty-five studies were retained for full-text analysis, while 489 papers were excluded, either because they were out of scope, or were not empirical studies. A Cohen’s κ coefficient of .878 was obtained with regard to inter-reviewer agreement in the assessment of titles and abstracts, indicating an almost perfect agreement between reviewers (Landis & Koch, 1977).
After full-text analysis, 45 studies were further excluded for the following reasons: publication before 1990 (n = 9); sample comprising patients in coma or in vegetative persistent state (n = 3); sample with subjects under 18 years old (n = 2); without intervention description (n = 8); use of electrostimulation (n = 3); research with focus on motor stimulation or other than sensory stimulation (n = 17); without the outcomes of intervention (n = 3). Thus, 23 papers fulfilled the eligibility criteria in the systematic review process (Figure 1).
Studies Characteristics
The analysis of the methodological quality of the studies was performed according to the classification of Cicerone et al. (2000). Class I was given to nine studies with a prospective design, randomized, and controlled. Two studies presented a prospective design, quasi-randomized to treatment conditions, and were classified as Class Ia. Class II comprised three prospective, non-randomized, cohort studies, or clinical studies enabling group comparisons according to different treatment conditions. Nine studies were classified as Class III, since they constitute clinical series without control group.
The reviewed studies included 621 participants (M = 27.0; SD = 17.8; Min = 7; Max = 69), of which 194 were female (31%). Study #17 only states that two participants in the experimental group were female. Participants’ age ranged between 19 and 89 years. Stroke was more frequent (n = 18 studies; 78%) than TBI (n = 5 studies; 22%).
Studies were published in 15 different journals. Journals with higher number of records included in this review were Neurorehabilitation and Neural Repair (n = 6) and Topics in Stroke Rehabilitation (n = 3).
Intervention Characteristics
The sensory stimulation programs of the reviewed studies were initiated 1 month to 8 years after the lesion. The only exception is study #23, which included participants with more than 8 years over the lesion. Study #21 did not provide this information (Table 1).

Multisensory stimulation programs were applied in 17 studies. Vision is the most frequently stimulated sense (n = 13; studies #1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13), followed by touch (n = 2; studies #15, 16). Vestibular function (n = 1; study #14), proprioception (n = 1; study #17), smell (n = 1; study #20), and hearing (n = 1; study #21) were also stimulated. Four multisensory stimulation programs were included, two directed to touch and proprioception (studies #18 and 19), one for vision and touch (study #22), and other for vision and hearing (study #23).
The frequency of stimulation ranged between twice-a-week to twice-a-day. This information was not provided in seven studies (#2, 7, 17, 20, 21, 22, 23).
The average duration of sessions was 41.75 min (SD = 16.09). Three studies did not provide this information (studies #2, 10, 17) and three studies reported an interval of duration (studies #16, 18, 19).
The intervention length ranged between 2 days (study #22) and 13 weeks. One study (#12) reported the intervention length in hours (M = 15.17 ± 1.1). This information was not provided in seven studies (#1, 7, 15, 17, 20, 21, 23).
The intervention programs ranged between 1 session (studies #7, 23) and 30 sessions (studies #8, 19). The number of sessions was not considered in seven studies (#3, 4, 5, 6, 11, 13, 14).
Intervention Method and Outcomes
In regard to visual stimulation, study #1 comprised restoration and compensation methods and occupational therapy. Compensation methods were used in six studies (#3, 5, 6, 7, 8, 11), virtual reality was used for compensation purposes in three (studies #6, 7, 11), four of them aimed to treat hemineglect by using top-down (studies #6, 7, 8) or bottom-up and top-down approaches combined (study #11).
Restoration method was employed in one study (#4).
Substitution method was used in one study (#9) and other compared the results of substitution method with those of compensation method in the treatment of hemineglect (study #10). In accordance to Marshall (2009), these approaches can be classified as bottom-up.
Five studies applied sensory and cognitive stimulation (#2, 12, 13, 20, 21); substitution methods were also used in two studies (#2, 12).
Concerning restoration, Mödden and collaborators (2012) showed different colored targets at different positions from the visual field limit without ocular movement. Bergsma and colleagues (2014) used a computerized program for visual restoration.
For compensation Mödden and collaborators (2012) used the exploration task from the Rehacom (HASOMED GmbH, Magdeburg, Germany), where patients were instructed to follow a moving ring and to identify a target-stimulus by pressing a key. The compensation of the visual field in the study by Nelles and colleagues (2001) was performed by using a square with red lights and both sides inward oriented. Head movements were not allowed. In the A condition patients had to stare to a point in the middle of the square; in condition B they had to perform exploratory eye movements in order to identify target-stimuli. Nelles et al. (2010) focused only on task B. The condition B led to an improvement in the detection of stimulus in the left and right hemianopia (p = .02; p = .003) and shorter reaction time in the hemianopic visual field (p = .02), kept in the 8 months follow-up assessment (Nelles et al., 2001) in stroke patients. In addition, it was reflected in the activation of the extra-striate cortex in the contralesion hemisphere (p < .001), which was maintained after training and 1 month follow-up (Nelles et al., 2010).
Compensation and restoration methods showed positive outcomes in hemianopia and visual field enlargement (p = .013 and p = .003, respectively), visual exploration (p = .003 and p = .005) and in attention (p = .001 and p = .033). Only the compensation method resulted in improvements in reading performance (p = .016) and visual conjunction search (p = .001), being considered the treatment of choice for the neglect (Mödden et al., 2012). Bergsma and colleagues (2014) also showed the efficacy of visual restoration in visual field enlargement (p < .005).
Virtual reality was employed: (1) to simulate a crossroad environment (Katz et al., 2005); (2) to present an visual identification task where objects flash from right to left at a distance and a touch task at close range with a virtual hand (Yasuda et al., 2017); (3) to use a robot arm in direction of target-stimuli creating haptic sensation (Dvorkin et al., 2013); (4) to multisensory stimulation using program RehAtt (Brain Stimulation AB, Holmsund, Sweden). RehAtt contemplated three tasks: (a) mental rotation, requiring moving figures from left to right side; (b) visual motor exploration, by allocating 3D cubes in three directions; (c) visual spatial and search task, aiming to move or fix moving targets with increasing speed (Fordell et al., 2016).
Participation in visual scanning training resulted in significant improvements in hemineglect, assessed with Mesulam Cancellation (Mesulam, 1985) (p < .01) and less time spent at the second assessment moment, but the percentage of improvements in time to complete the test was two times higher in the virtual reality group in comparison to the control group who received conventional visual scanning training. There were significant improvements in the activities of daily living in both groups (p < .05), but in the virtual reality group the numbers of times participants looked to the left was higher (p < .05) and accidents were fewer (p < .035) than in the control group, in the virtual reality environment. Moderate correlations were found between the decision time for real street crossing in the post-intervention and the activities of daily living, (r = −.49, p < .05) (Katz et al., 2005).
The second training led to improvements in the post-intervention assessment of hemineglect with Behavioural Inattention Test (Wilson et al., 1987) (p = .002) and respective subtests (all p < .003) (Yasuda et al., 2017).
In the second session with robot-rendered haptic stimulation, TBI participants took less pauses (p < .001), showed shorter reaction time (p = .007) and acquired more targets (p < .0001), compared to the first session (Dvorkin et al., 2013).
RehATT – scanning training for stroke patients with hemineglect resulted in significant post-intervention improvements in the Star cancelation test (Wilson & Halligan, 1987) (p = .006), Baking Tray Task (Tham, 1996) (p < .001), and Extinction test (Geeraerts et al., 2010) (p = .05). Post-intervention improvements were also found in the Catherine Bergego Scale (Bergego et al., 1995), both in self-report (p = .002) and observer assessment (p = .01), which were maintained in a 6-month follow-up assessment (p = .21) (Fordell et al., 2016).
The visuospatial training, also known as feedback based perceptive training, included tasks such as digits detection, drawings copy, reading and copying phrases, and image description (Piccardi et al., 2006; van Kessel et al., 2013). Hemineglect stroke patients received Visuospatial Scanning training for 3 days a week and combined treatment (Visuospatial Scanning training and lane tracking drive simulator) for 2 days a week during 3 weeks. Then, experimental condition received Visuospatial Scanning training and dual task and control condition continued with combined treatment for 3 weeks. Dual task consisted of a computerized visual reaction time task using virtual reality where the participants had to maintain their driving position while performing visuospatial scanning task. Participants in both groups had improvements between before and after training assessments in: Line cancelation (Albert, 1973) (p < .01); Letter cancelation (Diller & Weinberg, 1977), extrapersonal neglect (Zoccolotti et al., 1992) (all p < .001); personal neglect (Zoccolotti et al., 1992), subjective neglect (Towle & Lincoln, 1991), Bells test (Gauthier et al., 1989), Reading (all p < .005); Gray scales index (Tant et al., 2002) (p < .05). In addition both groups had improvements in lateral position in line tracking and in dual task – single detection task and lane tracking (all p < .05); omissions (p = .057) in the single detection task; reaction time to the left (p < .05) in single detection task; reaction time for middle (p < .05) and right stimuli (p = .058) on the previous mentioned dual task (van Kessel et al., 2013). Piccardi and collaborators (2006) also showed improvements in visuospatial exploration (Line cancelation, Letter cancelation, Bells test, Reading test) after Visuospatial training (p < .001), as well as increased functionality (semi-structured scale for the functional neglect assessment; Zoccolotti & Judica, 1991) (p < .011). The intervention had no effect on attention performance of stroke patients with hemineglect and attention disorders.
The adaptation of the prismatic googles is a substitution method in which the participants are placed in front of a semicircular desk and asked to point to three targets for improving hemineglect. In the study by Mizuno and colleagues (2011) the task was repeated with prismatic googles displacing the visual field 12° to the right. Brink, Visser-Meily, Schut, et al. (2017) used the same method to displace the visual field 10° to the right. The treatment of hemineglect also comprised psychoeducation and visual search tasks such as reading. Hemineglect patients who participated in this therapy obtained better results in the Functional Independence Measure (Uniform Data System for Medical Rehabilitation, NY, USA) between the post-intervention and follow-up, compared to controls who received the same treatment with neutral plastic glasses (p < .05). Specifically, the experimental group obtained higher scores in the Functional Independence Measure in the post-intervention (p < .05) and follow-up (p < .01), compared to the control group, as well as higher results in Behavioural Inattention Test – conventional test (Wilson et al., 1987) (p < .05) (Mizuno et al., 2011). Prismatic googles and sham adaptation with plain lenses also led to improvements in performance over time in the Catherine Bergego Scale, Mobility Assessment Course (Brink, Visser-Meily, & Nijboer, 2017), and static shape cancelation task (p < .001). However, the prismatic googles adaptation did not show greater effect than the simulated adaptation (Brink, Visser-Meily, Schut, et al., 2017).
Funk and colleagues (2013) implemented a perceptive training based on feedback through a computerized visual discrimination task concerning line orientation with stroke patients. In this task two oblique lines were presented and then rotated until the participants considered that they had the same orientation. A monocular training program based in the same task was also implemented, with the participants wearing an eye-patch to test if there was transfer of training from one eye to the other. This training had effects on constant errors and uncertainty intervals (both p < .001) in post-training compared to baseline, and the effects for 45º and 135º orientations were maintained at follow-up (p > .40). Furthermore, a decrease in the number of errors in the post-training compared with baselines were found in the Judgment of Line Orientation Test (Benton et al., 1983) and clock reading, as well as smaller deviations in a horizontal line and increase in the number of correct items in Marck-Levine test (Mack & Levine, 1981) (all p < .01) (Funk et al., 2013).
A platform named Guttman-NeuroPersonalTrainer (BrainHealth Solutions SL, Badalona, Spain), was used for the cognitive training of attention, memory, and executive functioning. The cognitive training was applied as usual, and with right eye-patching for visuospatial neglect. This training led to improvements in the Line bisection task (Schenkenberg et al., 1980) (p = .039), and combined with right hemifield eye-patching resulted in improvements both in the Line bisection task and in the Bells test (all p = .043). In the pre- and post-intervention comparison between stroke groups, patients who received the combined treatment had better performance in the Reading Task (p = .048), than patients only received cognitive training (Aparicio-Lopez et al., 2015).
The Cawthorne-Cooksey exercises (Cooksey, 1946) were used by Dai and colleagues (2013) to vestibular rehabilitation of stroke patients with hemineglect. These exercises encompass several steps: (a) to move the head up and down and to both sides with eyes open; (b) same as previous with eyes shut; (c) same as previous while looking at a moving target. It was observed an interaction between the group (experimental, control) and results at the 2nd and 4th weeks of intervention of the Behavioural Inattention Test – conventional test (p = .011; p = .009), Functional Independence Measure (p = .011; p = .011) and Postural Assessment Scale for Stroke patients (Benaim et al., 1999) (p = .001; p = .003) (Dai et al., 2013).
A somatosensory discrimination method was used in four studies (#15, 16, 17, 18, 19) and a bottom-up approach for the stimulation of proprioception was used in one study (#17).
The discriminative training used touch and involved the exploration and the discrimination of an unusual texture among other textures (Carey et al., 1993, 2016). A proprioceptive discriminative training as also applied, requiring participants to identify the perceived position of the fist in several predefined angles (Carey et al., 1993). After these training, stroke patients with impairment in proprioception and/or tactile discrimination revealed improvements in the affected hand (p = .003), resulting in comparable performance to the normal hand (Carey et al., 1993; Carey & Matyas, 2005).
Carey and Matyas (2005) followed the procedure of Carey and collaborators (1993) with texture grids, but other materials like glass, paper, leather, rubber and superficies with different degrees of roughness were used in order to favor stimuli generalization. Participants who performed this training revealed transference of the results to untrained related stimulus within the same modality (Carey & Matyas, 2005).
Besides the texture and limb position, the tactile recognition of objects focused on shape, size, weight, hardness and temperature were other tasks included in somatosensory discrimination training (Carey et al., 2011). The non-specific repeated exposure to sensory stimulus with diversified characteristics was performed through grasping of common objects and passive movements of the upper limb (Carey et al., 2011). After this training, stroke patients with impaired texture discrimination, limb position sense, and/or tactile object recognition obtained better results in sensory discrimination, compared to those who received repeated exposure to sensory stimulus (p = .004). The improvements were maintained 6 weeks and 6 months after the intervention (Carey et al., 2011).
The proprioceptive stimulation was also performed through a choice-reaction-time task. This task involved digit discrimination, and pressing a button with two different fingers in the case of even or odd numbers. This task was also performed with the vibration of the left extensor radialis muscle during the presentation of stimulus to study its effects on cognitive processes. TBI patients presented a higher number of correct detections of non-masked stimulus (p = .001) and in the vibration condition (p = .001). Compared to the healthy control group, they presented a lower hit rate of targets (p < .01) and longer reaction time (p < .0001). The experimental group showed shorter reaction times to target stimuli in the vibration condition, compared to the condition without vibration (p = .01). The latency of the P300, a brain potential evoked by vibratory stimuli, in this case, was higher in the experimental group in the condition without vibration (p = .01), compared to the controls. The experimental group showed normal performance on the Wechsler Adult Intelligence Scale – Revised (Wechsler, 1981), Recurring Figures Test (Rixecker & Hartje, 1980), Auditory Verbal Learning Test (Rey, 1958) and d2 (Brickenkamp & Zillmer, 1998), despite moderate cognitive slowness and impairment on executive functions (Müller et al., 2002).
The olfactory stimulation was performed through modified oxygen masks, which delivered peppermint fragrance in the experimental condition and unscented air in the control condition. This stimulation was provided during an attention task, in which pairs of lines with different distances from a central dot should be detected (Sullivan et al., 1998). Brain injury participants presented a detection rate similar than revealed by the healthy control group, but obtained a higher false alarm percentage in the unscented air condition (Sullivan et al., 1998).
The selective attention tasks (simple target detection and palindromes detection) of the Attention Process Training Program (Sohlberg & Mateer, 1987) were modified by manipulating background noise to improve selective attention using auditory stimuli, with an increase of decibel level along training. For the adaptive condition, the progression of noise was based on cognitive performance and in non-adaptive condition, participants did not adjust to the increasing noise, but were exposed to each noise level for the same period as the previous condition (Dundon et al., 2015). In the TBI group (adaptive and non-adaptive training) had positive effects on a dichotic listening task (p = .003), while in a healthy control group performance did not change over time (p = .34). Considering post-intervention assessment, in the Elevator Counting (Robertson et al., 1994) the control group obtained better results than the adaptive group (p = .000) and non-adaptive group (p = .036). The experimental group presented better results in post-intervention in the Elevator Counting with distraction and with reversal (p = .002 and p = .059), Telephone Search (Robertson et al., 1994) (p = .009), Rivermead Behavioral Memory Test – immediate and delayed recall (Wall et al., 1994) (p = .002 and p = .000), compared to the pre-intervention assessment. The performance in the post-training dichotic listening task correlated with the results obtained in the Perceived Stress Questionnaire (Cohen et al., 1983) (R2 = .50, p = .002), distractibility questionnaire (R2 = .32, p = .018) and with Calgary symptoms of somatic stress (Carlson & Thomas, 2007) (R2 = .28, p = .030) in pre-training. The amplitude of P3b, a subcomponent of the P300, increased from the pre- to post-intervention assessment in the adaptive group (p = .007) and non-adaptive group (p = .041) in response to infrequent targets in an oddball paradigm. The same did not happen in the control group (p = .598) (Dundon et al., 2015).
Snoezelen multisensory stimulation was used in one study (#23). The intervention comprised auditory and visual stimulation with colored bubble tubes, optic fiber bundles, rotating mirror ball, projector display, and sounds of nature (Gómez et al., 2016). Participation in one Snoezelen session led to a decrease in the median frequency (frequency that comprises 50% of the power spectral density) recorded by Encephalography (p = .04) in central and right temporal electrodes, suggesting deceleration of oscillatory activity (Gómez et al., 2016).
Assessment Procedures
Most studies (n = 10) followed a pre-and post-intervention design (studies #1, 4, 6, 7, 8, 12, 13, 16, 21, 23). Five studies conducted pre/post-intervention and follow-up assessments (studies #2, 3, 5, 9, 11). The assessment was performed only during the intervention in two studies (#17, 22) while in other two, it was conducted an assessment previously to the intervention, during the intervention, and at follow-up (n = 2; studies #18, 19). Other studies encompassed the following assessment moments: pre- intervention and during the intervention (n = 1; study #20); pre-intervention, during the intervention and post-intervention (n = 1; study #14); pre-intervention, during the intervention and at follow-up (n = 1; study #15); pre-intervention and during the intervention (n =1; study #10).
The follow-up assessment was conducted from 4 weeks (study #5) up to 8 months (study #3) after the end of the intervention.
In the studies included in this review, sensory stimulation programs focus on different sensory modalities and cognitive functions. Hence, the authors considered different outcome variables.
Attention was considered in six studies and evaluated through the: Test Battery of Attentional Performance (Zimmermann & Fimm, 2002) (study #1); reaction time between stimulus presentation and response (study #3); Computerized Screening Battery of Attentional Disorders (Zimmermann & Fimm, 1995) (studies #13, 17); d2 (study #17); percentage of correct detections and false alarm (study #20); Test of Everyday Attention (Robertson et al., 1994) (study #21); and dichotic listening (study #21).
Five studies considered the dysfunction and severity of ABI, using the National Institutes of Health Stroke Scale (Brott et al., 1989) (studies #11, 15, 16), the Stroke Impairment Assessment Set (Chino et al., 1996) (study #9), and the Glasgow Coma Scale (Teasdale & Jennett, 1974) (study #20).
Four studies presented an evaluation of the global cognitive functioning through the Mini Mental State Examination (Folstein et al., 1975) (studies #9, 10, 11) and the Rancho Los Amigos scale (Hagen et al., 1979) (study #22).
One study tested the premorbid intelligence quotient with Wechsler Adult Intelligence Scale – Revised (study #17). Memory was assessed through the Recurring Figures Test (study #17), Auditory Verbal Learning Test (study #17), and Rivermead Behavioral Memory Test (study #21).
Visuoconstructive and perceptive abilities were considered in study #2. Visual orientation was assessed with the computerized visual-spatial perception program (Kerkhoff & Marquardt, 1995), the visuospatial ability with the Judgment of Line Orientation Test, the visuoconstruction with the Mack-Levine test, the visuoperception with the analog clock reading task, and the spatial contrast sensitivity with the Cambridge Low Contrast Gratings (Wilkins et al., 1988) (study #2).
Hemineglect was a widely considered variable (studies #1, 2, 3, 6, 7, 8, 9, 10, 11, 12, 13, 14). The instrument that gathered more consensus to assess this condition was the Behavioural Inattention Test. This test or some of its scales were used in nine studies (#1, 6, 7, 8, 9, 10, 12, 13, 14). Three studies used Bells test (studies #8, 12, 13), two studies used Baking Tray Task (studies #8, 12). Two studies used assessment protocols adjusted to certain task (studies #6 and 8). Specifically, study #6 considered the individuals’ performance when crossing streets both in virtual reality environment and in the real streets. In study #8 the performance was assessed in a driver simulator. One study used the Virtual Reality – DiSTRO (Brain Stimulation AB, Holmsund, Sweden) (study #11), a hemineglect assessment battery in virtual reality. Other studies used were the Mesulam Symbol Cancellation test (study #6), Gray scales (study #8). Hemineglect was also assessed through: discrimination tasks on large visual displays (study #2); response to visual stimulus (study #3); shape cancelation (study #10); Figure Copying of Ogden (Ogden, 1985) (study #12); detection of targets on a dynamic task with Mobility Assessment Course (study #10). Finally, in study #8, the extrapersonal and personal neglect were assessed through a semi-structured scale and a subjective neglect questionnaire.
Reading was considered in five studies aimed at stimulating vision (studies #1, 2, 8, 12, 13), and considered a measure of hemineglect in three studies (#8, 12, 13). It was assessed through reading task (studies #2, 8, 12), subtests of the Wechsler Memory Test – Revised (Wechsler, 2004) (study #1), and reading of words and non-words task (study #13).
One study assessed the spatial dysgraphia through horizontal writing task (study #2).
The assessment of the visual field was considered in three studies, which administered the: Tubinger automatic perimeter (study #3); Dynamic Goldmann perimetry (study #4); and an automatic computerized system (study #5).
One study evaluated smell through the anosmia screening test (International Flavors and Fragrances Corporation, New York, United States of America) (study #20).
The somatosensory deficits were considered in four studies (#15, 16, 18, 19). For touch and texture discrimination or tactile recognition, the following assessment instruments were used: the Tactile Discrimination Test (Carey et al., 1997) (studies #15, 16, 18, 19); the Weinstein Enhanced Sensory Test (Weinstein, 1996) (studies #15, 16); the Fabric Matching Test (Carey, 1995) (studies #15, 19); the functional Tactile Object Recognition Test (Carey, 2006) (studies #15, 16); and the Grid Matching Test (Carey, 1995) (study #19). The Roylan hot and cold discrimination kit (studies #15, 16) was used for the temperature discrimination. Proprioception was assessed through the Wrist Position Sense Test (Carey et al., 1996) (studies #15, 16, 19), the Finger Position Sense (Carey, 1995) (study #15) and the Proprioceptive Discrimination Test (Carey et al., 1993) (study #18).
One study directed to the proprioceptive stimulation used a task-adjusted evaluation protocol, which included the reaction time to targets, the percentage of correctly detected targets, hits, misses, and false alarms (study #17). A task-adjusted evaluation protocol was also used in study #22, which considered spatial and temporal kinematic parameters of the hand movement (total trial time, hand path, velocity and distance from the target, duration of pauses, and number of targets acquired).
The analysis of brain structure and functioning was considered in seven studies (#4, 5, 13, 15, 16, 17, 21, 23). It was assessed through functional Magnetic Resonance Imaging (studies #4, 5, 16); Magnetic Resonance Imaging and Computerised Axial Tomography (studies #13, 15); and Event-related Potentials (studies #17, 21); and Eletroencephalography (study #23).
Independence in activities of daily living was considered in eight studies (#1, 4, 6, 9, 10, 11, 14, 15). It was assessed with the: conventional or extended Barthel Index (Mahoney & Barthel, 1965) (studies #1, 10, 11, 15); Functional Independence Measure (studies #6, 9, 14); Activities of Daily Living checklist (study #6); Functional Ambulation Categories (Holden et al., 1984) (study #10); Goal Attainment Scaling (Kiresuk & Sherman, 1968) (study #4). The impact of the hemineglect on activities of daily living was assessed with the Catherine Bergego Scale (studies #9, 10, 11, 12). Unspecified instruments were used in two studies (#4, 13).
Other variable assessed were handedness – Annett Questionnaire (Annett, 1970) (study #15).
Discussion
ABI can lead to sensory and cognitive deficits, impaired ability to explore the environment and, consequently, the lower functionality in activities of daily living. Despite the recognized impact of sensory deficits, the literature has focused on motor rehabilitation in stroke and sensory stimulation in altered states of consciousness in TBI. The sensory stimulation in stroke and mild to moderate TBI has deserved low interest of the literature. Therefore, the relationship between dose-response to sensory stimulation and clinical predictors need to be studied to adjust neurorehabilitation programs in ABI (Sullivan & Hedman, 2008).
The main objective of this review was to produce knowledge on the methodological characteristics of sensory stimulation programs in ABI and its results.
For the reasons described above, we did this review with the purpose of answering the following questions about the characteristics of the intervention: (RQ1) how long after the injury the sensory stimulation usually is initiated?; (RQ2) what are the most frequently used methods and what senses are stimulated?; (RQ3) what is the frequency, duration and average number of sessions and length of sensory stimulation programs?
Regarding the intervention results, we intend to answer the following questions: (RQ4) which assessment instruments are frequently used to evaluate the efficacy of sensory stimulation?; (RQ5) what are the immediate and long-term outcomes?; and (RQ6) are the outcomes of the stimulation programs generalized to cognitive functioning or activities of daily living?
Thus, a systematic review of literature was conducted in accordance with the principles of PRISMA (Moher et al., 2009; Shamseer et al., 2015) and Cochrane Collaboration’s recommendations (Higgins & Green, 2011).
Concerning the methodological quality of the revised studies, less than half of them were included in Class I. This means that most of the studies have methodological limitations and reported findings do not provide robust evidence.
Regarding the sample characteristics, there was a higher prevalence of stroke patients and males.
Answering RQ1, sensory stimulation was initiated within 12 months after the injury in most studies.
Considering the most frequently used methods and stimulated senses (RQ2), we found that sensory stimulation programs were mostly unisensory and the most frequently stimulated sense was vision. No program included taste stimulation. The most frequently used method in vision stimulation was compensation, with computerized tasks (Bergsma et al., 2014; Mödden et al., 2012) and boards for stimuli detection (Nelles et al., 2001, 2010). In visual substitution the most used method was the prismatic lens adaptation (Brink, Visser-Meily, Schut, et al., 2017; Mizuno et al., 2011). The hemineglect treatment was accomplished mainly with top-down approaches, and using virtual reality (Fordell et al., 2016; Katz et al., 2005; Yasuda et al., 2017). Sensory and cognitive stimulation were applied in a small number of studies, which used computerized tasks, among others (Aparicio-Lopez et al., 2015; Funk et al., 2013) and a program based on Attention Process Training (Dundon et al., 2015). Regarding somatosensory stimulation, the most frequently used methods were texture discrimination training and proprioceptive discrimination training (Carey & Matyas, 2005; Carey et al., 1993, 2011, 2016).
Concerning RQ3, studies are not consensual about the frequency, duration, number of sessions and length of sensory stimulation programs, and not all reported this information. Most frequent data were: stimulation frequency of three times a week; session duration of 30 min; intervention duration of 4 weeks; intervention length between 10 and 15 sessions.
In the majority of the studies, a pre- and post-intervention assessment design was applied. However, follow-up assessments were conducted in less than half of the studies.
With regard to assessment instruments (RQ4), we concluded that they were diversified, because of many variables considered. Hemineglect was the most widely considered variable and the most used instrument was Behavioural Inattention Test. The cognitive domain most assessed in pre- and post-intervention moments was attention, but all studies used different assessment instruments. In visual function assessment, sophisticated instruments were used that required specialized technical knowledge. In the assessment of somatosensory deficits, the most frequent instruments were the Tactile Discrimination Test and Wrist Position Sense Test. The ABI severity assessment was reported in a small number of studies, in spite of being considered central to neurorehabilitation program planning (Ślusarz et al., 2015). Assessments of general cognitive function, memory, and executive functions were performed in a small number of studies, mostly with the purpose of sample characterization. The structure and brain functioning were analyzed only in one third of the studies, using several techniques: magnetic resonance imaging; functional magnetic resonance imaging; computerized axial tomography; and event-related potentials. The use of different instruments and assessment protocols depending on the intervention programs prevents the comparison of their efficacy.
The included studies focused their assessment in the sensory modality targeted by the intervention. However, notions that sensory processing in the brain is unimodal and activity of a sensory system has little influence on others have been contradicted (Baier et al., 2006). For example, when auditory and visual stimuli are randomly combined, the sensory system relevant to the ongoing task is activated and the other is suppressed (Baier et al., 2006) showing an interaction between sensory modalities. However, when there is an association between auditory and visual information, both systems are activated. According to Theeuwes and collaborators (2007), we have different resources for perception, one for vision and other for auditory, but at a cognitive level, particularly in working memory, there is no separation. Thus, we considered important to include a battery of cognitive tests in the evaluation of programs efficacy.
Neuropsychology shows a growing concern regarding the development of assessment instruments and rehabilitation programs with ecological validity. This can be defined as the relationship between cognitive performance in neuropsychological assessment and behavior in activities of daily living (Spooner & Pachana, 2006). Ecological validity may be enhanced through the inclusion of functionality measures in real life environments. Few of the considered studies used instruments as the Test of Everyday Attention and the Catherine Bergego Scale, which enable to understand the impact of the interventions on participants’ ability to perform daily tasks. In this context, virtual reality is a methodology with potential to approximate the programs to the real contexts, hence allowing to reproduce the activities of daily living functioning (Parsons, 2015). Virtual reality can be useful in assessment and intervention, since it allows the accuracy of laboratory measures, through controlled and precise presentation of stimuli, with content similar to real life (Abbate et al., 2014; Parsons, 2015).
Affective disorders are common in ABI (Hackett et al., 2005; van Reekum et al., 2000), but none of the studies included measures of such disorders, even if multisensory stimulation has demonstrated a positive effect on depression and anxiety (Ozdemir & Akdemir, 2009).
Quality of life assessment is also used as an indicator of rehabilitation efficacy (Neznanov & Petrova, 2002). Again, none of the studies assessed this parameter.
Regarding the outcomes of the intervention programs (RQ5), Class I studies showed evidence of immediate improvement of vision (hemianopia) with compensation method (Mödden et al., 2012), hemineglect using vestibular intervention – Cawthorne-Cooksey exercises (Dai et al., 2013), cognitive functioning using proprioceptive stimulation – muscle vibration (Müller et al., 2002), sustained attention using olfactory stimulation (Sullivan et al., 1998) and an adapted version of Attention Process Training Program (Dundon et al., 2015). Immediate and long-term improvements were found on somatosensory discrimination using this intervention (Carey et al., 2011) and hemineglect using Guttman-NeuroPersonalTrainer with and without eye-patching (Aparicio-Lopez et al., 2015). Only one study of Class III used a stimulus-generalization training. However, studies of Class I showed generalization (RQ6) of compensation method effects to reading task (Mödden et al., 2012), prism adaptation therapy to functionality (Mizuno et al., 2011), Prismatic googles and sham adaptation with plain lenses to activities of daily living (Brink, Visser-Meily, Schut, et al., 2017), Cawthorne-Cooksey exercises to functionality (Dai et al., 2013), selective attention training with auditory stimulation to immediate and delayed recall (Dundon et al., 2015).
The studies of efficacy of prismatic lens in the subacute period of stroke showed contradictory results. Mizuno and collaborators (2011) showed an improvement, with maintenance of results in follow-up, while Brink and collaborators (Brink, Visser-Meily, Schut, et al., 2017) found that prismatic lens adaptation had not greater effect than simulated adaptation.
The studies included in this review have several limitations, such as: (a) small samples in half of the studies (less than 30 participants); (b) lack of a control group in 9 studies; (c) lack of randomization procedures in 12 studies; (d) lack of follow-up to examine the maintenance of the results in 15 studies; (e) small number of intervention sessions; (f) implementation of conventional rehabilitation simultaneously to the intervention being studied, producing confounding effects.
This literature review also has limitations. Only papers in English language and published after 1990 were included. Anyhow, it is worth mentioning that most of the studies that met the criteria had been published in the after 1990.
From this review, future studies on sensory stimulation in ABI should considered: (a) better sample characterization, for example, regarding educational level, ABI severity, biological variables as handedness, and pharmacological treatment; (b) performing cognitive assessment pre- and post-intervention; (c) using of an expanded assessment battery of sensory functioning, not only directed to the target sensory modality, due to interactions between sensory modalities; (d) measures of functionality, quality of life, affective disorders and emotional well-being, to enhance the ecological validity of the protocols; (e) performing follow-up assessments, to provide information about the maintenance of intervention outcomes; (f) inclusion of active and passive control groups; (g) whenever the intervention programs begin in the first months after the injury control groups are paramount to dissociate the spontaneous recovery from the effects of the rehabilitation programs; (h) using structural and functional brain measures to provide neurobiological evidence of intervention efficacy.
Considering the findings and shortcomings mentioned above, this review suggests that sensory stimulation has positive outcomes in ABI, including at cognitive and affective levels, with potential benefits to patients functionality in activities of daily living, but future studies may consider methodologies that provide stronger evidence, enhance the generalization of outcomes, and allow comparative analysis between studies. The recommendations presented here are important to achieve these goals.
Joana Pinto has a master’s degree in clinical neuropsychology and specialization in Clinical and Health Psychology. Currently, she is a PhD student at Laboratory of Neuropsychophysiology with a project in cognitive and affective neurosciences field. Her main research interests includes neurocognitive rehabilitation, and neurodegenerative diseases.

Artemisa R. Dores (PhD in cognitive neurosciences) is a specialist in Clinical and Health Psychology, and in Neuropsychology. Artemisa R. Dores is Professor at the Polytechnic of Porto, School of Health, and member of the Rehabilitation Research Center (ESS – P. Porto) and of the Neuropsychophysiology Laboratory (FPCEUP).

Bruno Peixoto holds a Doctorate degree in Psychology (Psychobiology) and advanced specialization in Neuropsychology. He is Professor and researcher at CESPU, University Institute of Health Sciences in the fields of Neuroanatomy and Neuropsychology. His main research interests are dementia and neuropsychology of medical conditions. He is a research collaborator with CINTESIS.

Andreia Geraldo is a specialist in clinical and health psychology, with pre-specialization in neuropsychology. She is a PhD student with a project within cognitive and affective neurosciences. Her main research interests involve the use of new ICTs across neuropsychological assessment and rehabilitation, ABI and other neurodegenerative disorders, and gambling/gaming.

Fernando Barbosa is a Clinical Psychologist with specialization in Neuropsychology and PhD in Neuroscience. He is Professor at the University of Porto, Portugal and Coordinator of the Laboratory of Neuropsychophysiology. He is Founding member of the European Society for Cognitive and Affective Neuroscience, and member of the Standing Committee on Clinical Neuropsychology of the European Federation of Psychologists’ Associations.

References
(2014). Sensory stimulation for patients with disorders of consciousness: From stimulation to rehabilitation. Frontiers in Human Neuroscience, 8(616), 1–5. https://doi.org/10.3389/fnhum.2014.00616
(1973). A simple test of visual neglect. Neurology, 23, 658–664. https://doi.org/10.1212/WNL.23.6.658
(1970). Classification of hand preference by association analysis. British Journal of Psychology, 61, 303–321. https://doi.org/10.1111/j.2044-8295.1970.tb01248.x
(2015). Cognitive rehabilitation with right hemifield eye-patching for patients with sub-acute stroke and visuo-spatial neglect: A randomized controlled trial. Brain Injury, 29(4), 501–507. https://doi.org/10.3109/02699052.2014.995230
(2006). Cross-modal processing in early visual and auditory cortices depends on expected statistical relationship of multisensory information. Journal of Neuroscience, 26(47), 12260–12265. https://doi.org/10.1523/JNEUROSCI.1457-06.2006
(1999). Validation of a standardized assessment of postural control in stroke patients: The Postural Assessment Scale for Stroke Patients (PASS). Stroke, 30(9), 1862–1868. https://doi.org/10.1161/01.STR.30.9.1862
(1983). Judgement of line orientation, Oxford University Press.
(1995). Validation d’une échelle d’évaluation fonctionnelle de l’héminégligence dans la vie quotidienne: L’échelle CB
[Functional consequences of unilateral neglect: Validation of an evaluation scale, the CB scale] . Annales de Réadaptation et de Médecine Physique, 38(4), 183–189. https://doi.org/10.1016/0168-6054(96)89317-2(2014). Visual daily functioning of chronic stroke patients assessed by goal attainment scaling after visual restorative training: An explorative study. Topics in Stroke Rehabilitation, 21(5), 400–412. https://doi.org/10.1310/tsr2105-400
(2012). Transfer effects of training-induced visual field recovery in patients with chronic stroke. Topics in Stroke Rehabilitation, 19(3), 212–225. https://doi.org/10.1310/tsr1903-212
(2013). Cognitive rehabilitation for spatial neglect following stroke. Cochrane Database of Systematic Reviews, 7, Article CD003586. https://doi.org/10.1002/14651858.CD003586.pub3
(1998). d2 Test of Attention, Hogrefe & Huber.
(2017). Dynamic assessment of visual neglect: The Mobility Assessment Course as a diagnostic tool. Journal of Clinical and Experimental Neuropsychology, 40(2), 161–172. https://doi.org/10.1080/13803395.2017.1324562
(2017). Prism adaptation in rehabilitation? No additional effects of prism adaptation on neglect recovery in the subacute phase poststroke: A randomized controlled trial. Neurorehabilitation and Neural Repair, 31(12), 1017–1028. https://doi.org/10.1177/1545968317744277
(1989). Measurements of acute cerebral infarction: a clinical examination scale. Stroke, 20(7), 864–870. https://doi.org/10.1161/01.STR.20.7.864
(2013). DeJong’s the neurologic examination (7th ed.). Lippincott Williams & Wilkins.
(1995). Somatosensory loss after stroke. Critical Reviews in Physical and Rehabilitation Medicine, 7, 51–91. https://doi.org/10.1615/CritRevPhysRehabilMed.v7.i1.40
(2006).
Loss of somatic sensation . In M. SelzerS. ClarkeL. CohenP. DuncanF. H. GageEds., Textbook of Neural Repair and Rehabilitation. Vol. II Medical Neurorehabilitation (pp. 231–247). Cambridge University Press.(2016). Same intervention–different reorganization: The impact of lesion location on training-facilitated somatosensory recovery after stroke. Neurorehabilitation and Neural Repair, 30(10), 988–1000. https://doi.org/10.1177/1545968316653836
(2011). SENSe: Study of the effectiveness of neurorehabilitation on sensation: A randomized controlled trial. Neurorehabilitation and Neural Repair, 25(4), 304–313. https://doi.org/10.1177/1545968310397705
(2005). Training of somatosensory discrimination after stroke: Facilitation of stimulus generalization. American Journal of Physical Medicine & Rehabilitation, 84(6), 428–442. https://doi.org/10.1097/01.phm.0000159971.12096.7f
(2018). Effects of somatosensory impairment on participation after stroke. American Journal of Occupational Therapy, 72, Article 7203205100. https://doi.org/10.5014/ajot.2018.025114
(1993). Sensory loss in stroke patients – effective training of tactile and proprioceptive discrimination. Archives of Physical Medicine and Rehabilitation, 74(6), 602–611. https://doi.org/10.1016/0003-9993(93)90158-7
(1996). Impaired limb position sense after stroke: A quantitative test for clinical use. Archives of Physical Medicine and Rehabilitation, 77, 1271–1278. https://doi.org/10.1016/s0003-9993(96)90192-6
(1997). Impaired touch discrimination after stroke: A quantitative test. Neurorehabilitation and Neural Repair, 11, 219–232.
(2007). Development of the Calgary Symptoms of Stress Inventory (C-SOSI). International Journal of Behavioral Medicine, 14(4), 249–256. https://doi.org/10.1007/BF03003000
(2000). Evidence-based cognitive rehabilitation: Recommendations for clinical practice. Archives of Physical and Medical Rehabilitation, 81(12), 1596–1615. https://doi.org/10.1053/apmr.2000.19240
(1996).
Stroke Impairment Assessment Set (SIAS) . In N. ChinoJ. L. MelvinEds., Functional evaluation of stroke patients (pp. 19–31). Springer.(1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396.
(2007). Sensory impairment and recovery after stroke [Doctoral dissertation, University of Nottingham]. University of Nottingham repository. http://eprints.nottingham.ac.uk/10247/1/PHD_final.pdf
(1946). Rehabilitation in vestibular injuries. Proceedings of the Royal Society of Medicine, 39, 273–278. https://doi.org/10.2307/2136404
(2013). Effects of primary caregiver participation in vestibular rehabilitation for unilateral neglect patients with right hemispheric stroke: A randomized controlled trial. Neuropsychiatric Disease and Treatment, 9, 477–484. https://doi.org/10.2147/ndt.s42426
(1977). Hemi-inattention in rehabilitation: The evolution of a rational remediation program. Advances in Neurology, 18, 63–82.
(2015). Impaired auditory selective attention ameliorated by cognitive training with graded exposure to noise in patients with traumatic brain injury. Neuropsychologia, 75, 74–87. https://doi.org/10.1016/j.neuropsychologia.2015.05.012
(2013). A “virtually minimal” visuo-haptic training of attention in severe traumatic brain injury. Journal of Neuroengineering and Rehabilitation, 10(92), 1–9. https://doi.org/10.1186/1743-0003-10-92
(2017). Global burden of stroke. Circulation Research, 120(3), 439–448. https://doi.org/10.1161/CIRCRESAHA.116.308413
(2011). Electrophysiological assessments of cognition and sensory processing in TBI: applications for diagnosis, prognosis and rehabilitation. International Journal of Psychophysiology, 82(1), 4–15. https://doi.org/10.1016/j.ijpsycho.2011.03.005
(1975). “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. https://doi.org/10.1016/0022-3956(75)90026-6
(2016). RehAtt – scanning training for neglect enhanced by multi-sensory stimulation in Virtual Reality. Topics in Stroke Rehabilitation, 23(3), 191–199. https://doi.org/10.1080/10749357.2016.1138670
(2013). Effects of feedback-based visual line-orientation discrimination training for visuospatial disorders after stroke. Neurorehabilitation and Neural Repair, 27(2), 142–152. https://doi.org/10.1177/1545968312457826
(1989). The Bells Test: A quantitative and qualitative test for visual neglect. International Journal of Clinical Neuropsychology, 11, 49–54.
(2010). Asynchronous stimulus presentation in visual extinction: A psychophysical study. Journal of Neuropsychology, 4(2), 167–179. https://doi.org/10.1348/174866409X485071
(2016). Characterization of EEG patterns in brain-injured subjects and controls after a Snoezelen® intervention. Computer Methods and Programs in Biomedicine, 136, 1–9.
(2018). Somatosensory stimulation to improve hand and upper limb function after stroke – a systematic review with meta-analyses. Topics in Stroke Rehabilitation, 25(2), 150–160. https://doi.org/10.1080/10749357.2017.1389054
(2005). Frequency of depression after stroke: A systematic review of observational studies. Stroke, 36(6), 1330–1340. https://doi.org/10.1161/01.STR.0000165928.19135.35
(1979). Rehabilitation of the head injured adult: Comprehensive physical management (pp. 87–89). Professional Staff Association of Rancho Los Amigos Hospital.
(2016). Past, present, and future of traumatic brain injury research. Neurosurgery Clinics, 27(4), 375–396. https://doi.org/10.1016/j.nec.2016.05.002
(2011). Cochrane handbook for systematic reviews of interventions. (Version 5.1.0). http://handbook.cochrane.org
(1984). Clinical gait assessment in the neurologically impaired: Reliability and meaningfulness. Physical Therapy, 64, 35–40. https://doi.org/10.1093/ptj/64.1.35
(2018). Noninvasive brain stimulations for unilateral spatial neglect after stroke: A systematic review and meta-analysis of randomized and nonrandomized controlled trials. Neural Plasticity, 2018, 1–25. https://doi.org/10.1155/2018/1638763
(2005). Interactive virtual environment training for safe street crossing of right hemisphere stroke patients with Unilateral Spatial Neglect. Disability and Rehabilitation, 27(20), 1235–1243. https://doi.org/10.1080/09638280500076079
(1995). VS: A new computer program for detailed offline analysis of visual-spatial perception. Journal of Neuroscience Methods, 63, 75–84. https://doi.org/10.1016/0165-0270(95)00090-9
(1968). Goal attainment scaling: A general method of evaluating comprehensive mental health programs. Community Mental Health Journal, 4(6), 443–453.
(1977). The measurement of observer agreement for categorical data. Biometrics, 33, 159–174. https://doi.org/10.2307/2529310
(2011). Does sensory transcutaneous electrical stimulation enhance motor recovery following a stroke? A systematic review. Neurorehabilitation and Neural Repair, 25(9), 799–809. https://doi.org/10.1177/1545968310397205
(2010). Dual sensory impairment (DSI) in traumatic brain injury (TBI) – An emerging interdisciplinary challenge. NeuroRehabilitation, 26(3), 213–222. https://doi.org/10.3233/NRE-2010-0557
(2016). Epidemiology of traumatic brain injury over the world: A systematic review. General Medicine: Open Access, 4(5), e275.
(2013). The effectiveness of different treatment modalities for the rehabilitation of unilateral neglect in stroke patients: A systematic review. NeuroRehabilitation, 33(4), 611–620. https://doi.org/10.3233/NRE-130986
(1981). The basis of visual constructional disability in patients with unilateral cerebral lesions. Cortex, 17, 515–532. https://doi.org/10.1016/S0010-9452(81)80059-7
(1965). Functional evaluation – the Barthel Index. Maryland State Medical Journal, 14, 61–65.
(2009). Rehabilitation approaches to hemineglect. The Neurologist, 15(4), 185–192. https://doi.org/10.1097/NRL.0b013e3181942894
Mesulam, M. M. (Ed.). (1985). Principles of behavioral neurology, Oxford University Press.
(2011). Prism adaptation therapy enhances rehabilitation of stroke patients with unilateral spatial neglect: A randomized, controlled trial. Neurorehabilitation and Neural Repair, 25(8), 711–720. https://doi.org/10.1177/1545968311407516
(2012). A randomized controlled trial comparing 2 interventions for visual field loss with standard occupational therapy during inpatient stroke rehabilitation. Neurorehabil Neural Repair, 26(5), 463–469. https://doi.org/10.1177/1545968311425927
(2009). The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med, 6(6), Article e1000097. https://doi.org/10.1371/journal.pmed1000097
(2002). The effects of proprioceptive stimulation on cognitive processes in patients after traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 83(1), 115–121. https://doi.org/10.1053/apmr.2002.27472
(2001). Compensatory visual field training for patients with hemianopia after stroke. Neuroscience Letters, 306(3), 189–192. https://doi.org/10.1016/s0304-3940(01)01907-3
(2010). Eye-movement training-induced changes of visual field representation in patients with post-stroke hemianopia. Journal of Neurology, 257(11), 1832–1840. https://doi.org/10.1007/s00415-010-5617-1
(2002). Quality of life as a measure of the effectiveness of patient rehabilitation. International Journal of Mental Health, 31(1), 38–48. https://doi.org/10.1080/00207411.2002.11449542
(1985). Anterior-posterior interhemispheric differences in the loci of lesions producing visual hemineglect. Brain and Cognition, 4, 59–75. https://doi.org/10.1016/0278-2626(85)90054-5
(2009). Effects of multisensory stimulation on cognition, depression and anxiety levels of mildly-affected Alzheimer’s patients. Journal of the Neurological Sciences, 283(1–2), 211–213. https://doi.org/10.1016/j.jns.2009.02.367
(2016). Effectiveness of sensory stimulation to improve arousal and alertness of people in a coma or persistent vegetative state after traumatic brain injury: A systematic review. American Journal of Occupational Therapy, 70(3), 7003180030p1–7003180030p8.
(2015). Virtual reality for enhanced ecological validity and experimental control in the clinical, affective and social neurosciences. Frontiers in Human Neuroscience, 9(660), 1–19. https://doi.org/10.3389/fnhum.2015.00660
(2006). Efficacy of visuo-spatial training in right-brain damaged patients with spatial hemineglect and attention disorders. Cortex, 42(7), 973–982. https://doi.org/10.1016/s0010-9452(08)70203-x
(1958). L’Examen clinique en psychologie
[The psychological examination] . Presses Universitaires de France.(1980). Kimura’s Recurring‐Figures‐Test: A normative study. Journal of Clinical Psychology, 36(2), 465–467. https://doi.org/10.1002/jclp.6120360213
(1994). The Test of Everyday Attention: TEA, Thames Valley Test Company.
(2013). A prospective profile of visual field loss following stroke: Prevalence, type, rehabilitation, and outcome. BioMed Research International, 2013, 1–12. https://doi.org/10.1155/2013/719096
(1980). Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology, 30, 509–517. https://doi.org/10.1212/WNL.30.5.509
(2017). Sensory impairments and cognitive function in middle-aged adults. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 72(8), 1087–1090. https://doi.org/10.1093/gerona/glx067
(2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. British Medical Journal, 349, Article g7647. https://doi.org/10.1136/bmj.g7647
(2015). Measuring scales used for assessment of patients with traumatic brain injury: multicenter studies. Patient Preference and Adherence, 9, 869–875. https://doi.org/10.2147/PPA.S83551
(1987). Effectiveness of an attention-training program. Journal of Clinical and Experimental Neuropsychology, 9(2), 117–130. https://doi.org/10.1080/01688638708405352
(2006). Ecological validity in neuropsychological assessment: A case for greater consideration in research with neurologically intact populations. Archives of Clinical Neuropsychology, 21(4), 327–337. https://doi.org/10.1016/j.acn.2006.04.004
(1998). Effects of olfactory stimulation on the vigilance performance of individuals with brain injury. Journal of Clinical and Experimental Neuropsychology, 20(2), 227–236. https://doi.org/10.1076/jcen.20.2.227.1175
(2008). Sensory dysfunction following stroke: Incidence, significance, examination, and intervention. Topics in Stroke Rehabilitation, 15(3), 200–217. https://doi.org/10.1310/tsr1503-200
(2002). Grey scales uncover similar attentional effects in homonymous hemianopia and visual neglect. Neuropsychologia, 40, 1474–1481. https://doi.org/10.1016/S0028-3932(01)00197-X
(1974). The Glasgow Coma scale. Lancet, 2, 81–84. https://doi.org/10.1016/S1474-4422(14)70120-6
(1996). The Baking Tray Task: A Test of Spatial Neglect. Neuropsychological Rehabilitation, 6, 19–26. https://doi.org/10.1080/713755496
(2007).
Cross-modal interactions between sensory modalities: Implications for the design of multisensory displays . In A. F. KramerD. A. WiegmannA. KirlikEds., Attention: From theory to practice (pp. 196–206). Oxford University Press.(2016). Multisensory stimulation to improve low-and higher-level sensory deficits after stroke: A systematic review. Neuropsychology Review, 26(1), 73–91. https://doi.org/10.1007/s11065-015-9301-1
(1991). Development of a questionnaire for detecting everyday problems in stroke patients with unilateral neglect. Clinical Rehabilitation, 5, 135–140. https://doi.org/10.1177/026921559100500208
(2008). Sensory loss in hospital-admitted people with stroke: Characteristics, associated factors, and relationship with function. Neurorehabilitation and Neural Repair, 22(2), 166–172. https://doi.org/10.1177/1545968307305523
Turner-Strokes, L. (Ed.). (2003). Rehabilitation following acquired brain injury: National clinical guidelines, Royal College of Physicians.
(2013). Visual scanning training for neglect after stroke with and without a computerized lane tracking dual task. Frontiers in Human Neuroscience, 7(358), 1–11. https://doi.org/10.3389/fnhum.2013.00358
(2000). Can traumatic brain injury cause psychiatric disorders? The Journal of Neuropsychiatry and Clinical Neurosciences, 12(3), 316–327.
(1994). The extended Rivermead Behavioural Memory Test: A measure of everyday memory performance in normal adults. Memory, 2(2), 149–166. https://doi.org/10.1080/09658219408258942
(1981). Wechsler Adult Intelligence Scale – Revised, Psychological Corporation.
(2004). Wechsler Memory Test (WMS-R, revised version, 1987), Huber.
(1996). Hand instrument: The “Shirt-Pocket Portable” Nerve Tester. Care and use manual, North Coast Medical.
. (1996). The world health report 1996: Fighting disease, fostering development, http://www.who.int/entity/whr/1996/en/whr96_en.pdf?ua=1
(1988). Age-related norms for the Cambridge Low Contrast Gratings, including details concerning their design and use. Clinical Vision Science, 2, 201–212.
(1987). Behavioural Inattention Test, Thames Valley Test Company.
(1987). Development of a behavioral test of visuospatial neglect. Archives of Physical Medicine and Rehabilitation, 68, 98–102.
(2013). Rehabilitation interventions for unilateral neglect after stroke: A systematic review from 1997 through 2012. Frontiers in Human Neuroscience, 7(187), 1–11. https://doi.org/10.3389/fnhum.2013.00187
(2017). Validation of an immersive virtual reality system for training near and far space neglect in individuals with stroke: A pilot study. Topics in Stroke Rehabilitation, 24(7), 533–538. https://doi.org/10.1080/10749357.2017.1351069
(1947). Psychological aspects of rehabilitation in cases of brain injury. British Journal of Psychology, 37(2), 60–69. https://doi.org/10.1111/j.2044-8295.1947.tb01121.x
(1995). Test for Attentional Performance (TAP), Psytest (English version 1.02).
(2002). Testbatterie zur Aufmerksam – keitsprüfung (TAP),
[Test battery for Attentional Performance] . Psytest.(1992). Psychometric characteristics of two semi-structured scales for the functional evaluation of hemi-inattention in extrapersonal and personal space. Neuropsychological Rehabilitation, 2(3), 179–191. https://doi.org/10.1080/09602019208401407
(1991). Functional evaluation of hemineglect by means of semi-structured scale: Personal extrapersonal differentiation. Neuropsychological Rehabilitation, 1(1), 33–44. https://doi.org/10.1080/09602019108401378



