Plasminogen activator inhibitor-1 (PAI-1) 4G/5G promoter polymorphisms and risk of venous thromboembolism – a meta-analysis and systematic review
Abstract
Summary:Background: A 4G/5G polymorphism in the promoter region of the plasminogen activator inhibitor type 1 (PAI-1) gene has been reported to enhance the plasma levels of PAI-1, which plays an important role in fibrinolysis disorders and venous thromboembolism, but a large number of studies have reported inconclusive results. Therefore, we performed a meta-analysis to analysis these associations. Materials and methods: We performed a publication search for articles published before April 2019 by using the electronic databases of web of Science, Embase, PubMed, CNKI, CBM and WanFang data with the following terms “PAI-1”, “polymorphism”, “Venous Thromboembolism”. Two investigators independently extracted data and assessed study quality. Statistical analyses were undertaken using Stata 14.0. Results: A total of 27 studies, with 3135 patients and 5346 controls were included. Overall, the variant PAI-1 4G/4G and PAI-1 4G/5G was associated with venous thromboembolism risk, compared with the PAI-1 5G/5G allele in the populations included in the analysis. Stratified analysis revealed that PAI-1 4G/4G and PAI-1 4G/5G genotypes were associated with an increased VTE risk among Asia populations in all five genetic models. Conclusions: The PAI-1 4G/5G polymorphism may be a potential biomarker of VTE risk, particularly in Asia populations. Further larger studies with multi-ethnic populations are required to further assess the association between PAI-1 4G/4G polymorphisms and VTE risk.
Introduction
Venous thromboembolism (VTE) is a serious threat to life and health, commonly known as the silent killer [1]. Recent study has found that VTE is a polygenetic disease [2]. PAI-1 is a serine protease with negative feedback to fibrinolysis, activating and inhibiting coagulation. Thus, PAI-1 plays a role in dissolving blood clots by inhibiting fibrinolytic activators tPA and uPA, which not only inhibits fibrinolysis in blood vessels, but also participates in the regulation of cell adhesion and migration [3]. Several recent studies focused on the 4G/5G insertion/deletion polymorphisms in the PAI-1 gene promoter region, whose genetic polymorphism (known as 4G/5G polymorphism) is related to the plasma and activity level of PAI-1 [4]. There may be four (4G) or five (5G) guanosine residues in the 4G/5G polymorphism. Baglin T [4] found that 4G allele could bind to transcription activator, enhancing mRNA transcription and increasing the level of PAI-1. In contrast, 5G allele could bind to repressors, reducing mRNA transcription and the level of PAI-1. The 4G/4G homozygous genotype of PAI-1 is associated with the increase of the level of PAI-1, which leads to a state of low fibrinolysis, thereby increasing the risk of venous thromboembolism. However, the results of these observations remain controversial and inconclusive. In the present study, some studies showed that PAI-1 4G/4G presented a higher risk of venous thrombosis [5], while other studies did not find an association [6]. We conducted an updated meta-analysis in order to derive more precise and more comprehensive estimation of the associations between the PAI-1 4G/5G polymorphism and susceptibility to VTE.
Materials and methods
Literature screening and identification of relevant studies
We performed a publication search for articles published before April 2019 by using the electronic databases of web of Science, Embase, PubMed, CNKI, CBM and WanFang data with the following terms “PAI-1”, “polymorphism”, “venous thromboembolism”, by two independent investigators (YunRui Jin and Qiang Zhang). Hand searches were also performed to identify more eligible articles in the reference lists of included articles not retrieved by initial electronic search. We reviewed all retrieved articles by reading the titles and abstracts. Then, the full text of the possibly relevant studies was examined for further suitability evaluations in our present meta-analysis. The whole studies in the meta-analysis were firstly published in the primary literature with no reproduction in other studies. All studies matching the inclusion criteria were retrieved for further examination and data extraction. All of the investigators have received training in literature search, statistics and evidence-based medicine.
Inclusion criteria and exclusion criteria
Studies were included if: (1) Case-control studies evaluated the association between the PAI-1 4G/5G polymorphism and VTE risk, (2) patients diagnosed with VTE in the case group and healthy people in the control group, (3) detailed genotype data were provided for the calculation of odds ratio (OR) and 95 % confidence interval (CIs), (4) genotype frequency of the control group was consistent with the Hardy-Weinberg equilibrium (HWE), which is a common practice for quality control in genetic studies. P < 0.05 was considered representative of a departure from Hardy-Weinberg equilibrium. (5) if serial studies from the same group of people were reported, included the latest study and (6) Publication date and publication language were not restricted in our search. The following types of articles were excluded: (1) studies unrelated to PAI-1 polymorphism and VTE risk; (2) case studies, case series, intervention studies, qualitative studies, systematic reviews, abstracts, conference papers, meta-analysis and repetition of previous publications; (3) the same article of previous publications; (4) the information provided in the original literature is not enough to calculate the statistical index OR value.
Quality assessment and data extraction
Two investigators (YunRui Jin and Qiang Zhang) extracted data independently from the included studies using a standard protocol and data-collection form according to the above inclusion criteria and reached consensus on all items. Disagreements were resolved by discussion or consensus with a third reviewer (Mingfang Xu). The data extracted from eligible studies included the first author, year of publication, participant characteristics (i.e., mean age, sample size, ethnicity), genotyping method, total numbers of cases and controls, and genotype frequencies of cases and controls, etc.
The quality of the included studies was assessed by two independent reviewers (YunRui Jin and Qiang Zhang) according to the Newcastle-Ottawa Quality Assessment Scale (NOS) for case-control studies. Rating criteria were as follows: Low quality = 0~5; medium quality = 6~7; and high quality = 8~9.
Data synthesis and statistical analysis
OR corresponding to 95 % CI was used to assess the strength of association between PAI-1 4G/5G polymorphism and susceptibility to VTE risk. The heterogeneity of the study was tested by Q test and I2 statistics. If P < 0.1 or I2 ≥ 50 %, which indicates that there was heterogeneity among the research results, the random effect model was used for the combined analysis. Otherwise, use the fixed effect model. The stability and reliability of the meta-analysis was evaluated by sensitivity analysis. Egger’s and Begg’s test for the evaluation of potential publication bias. Pooled ORs were calculated for allele frequency comparison (4G versus 5G (allele model), 4G/4G + 5G/4G versus 5G/5G (dominant model), 4G/4G versus 5G/4G + 5G/5G (recessive), 4G/4G + 5G/5G versus 5G/4G (additive model), 4G/4G versus 5G/5G homozygote model and 5G/4G versus 5G/5G (heterozygote model)), respectively. All of the statistical tests were performed with software STATA 14.0 (STATA Corporation, College Station, TX, USA). All the P values are two-sided, and P < 0.05 served as the threshold of statistically significant.
Results
Study identification and characteristics of included studies
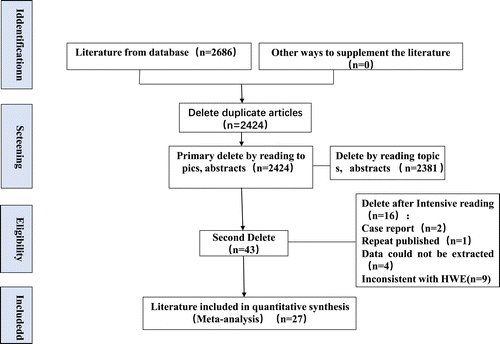
After first search in web of Science, Embase, PubMed, CNKI, CBM and WanFang databases, 2686 articles were retrieved. No more study was included after further manual search of reference lists. After importing into Endnote, 262 duplicate references were deleted. Full text of the remain 43 records were obtained after excluded for improper titles and/or abstracts. 16 studies were excluded (2 case reports, 1 repeat published study, 4 without detailed data, and 9 inconsistent with HWE studies) and finally 27 studies [7–33] involving a total of 3135 disease cases and 5346 controls met the inclusion criteria and were subjected to further examination (Figure 1). Characteristics of included studies are summarized in Table I, including ethnicity, age genotype detection method and thrombus type (including deep venous thrombosis (DVT), pulmonary embolism (PE), cerebral venous thrombosis (CVT), portal vein thrombosis (PVT).
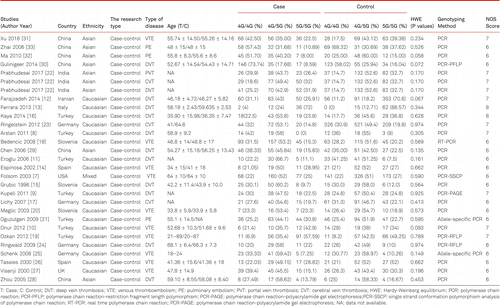
All studies were case–control studies, including 19 studies of Caucasian descents (7 Turks, 4 Germans, 3 Slovenian and 5 others), 7 studies of Asian descents (5 Chinese, 1 Indian, and 1 Iranians) and 1 study of mixed descent (USA). The departure of frequencies of PAI-1 4G/5G polymorphism from expectation under Hardy-Weinberg equilibrium (HWE) was assessed by the goodness-of-fit chi-square test in controls for each study. Several genotyping methods were employed in the studies including polymerase chain reaction (PCR), polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP), PCR-PAGE and allele-specific. NOS Scores were 6–8.
Quantitative synthesis
The association between PAI-1 4G/5G polymorphism and susceptibility to VTE was analyzed in 27 independent studies. The results are shown in the electronic supplementary material (ESM) 1, Table. Heterogeneity test results from the Meta-analysis showed that there was statistical heterogeneity among the results in all 6 genetic models, and the random effect model was applied to analyze the results. Significant association between PAI-1 4G/5G polymorphism and susceptibility to VTE was identified, when all the eligible studies were pooled (4G versus 5G (allele model): OR = 1.25, 95 % CI [1.05, 1.49], P = 0.014; 4G/4G + 5G/4G versus 5G/5G (dominant model): OR = 1.38, 95 % CI [1.06, 1.81], P = 0.019; 4G/4G versus 5G/4G + 5G/5G (recessive): OR = 1.34, 95 % CI [1.10–1.63], P = 0.004; 5G/4G versus 5G/5G (heterozygote model): OR = 1.27, 95 % CI [1.00–1.65], P = 0.049); 4G/4G versus 5G/5G (homozygote model): OR = 1.59, 95 % CI [1.17–2.15], P = 0.003).No significant association was found in additive model (4G/4G + 5G/5G versus 5G/4G: OR = 0.98, 95 % CI [0.83, 1.17], P = 0.856).
Subgroup analysis
Next, subgroup analyses were performed .7 out of the 27 included studies were conducted in Asian population. In ethnicity subgroup analysis, significantly increased risks were found in Asians in 5 genetic models (4G versus 5G (allele model): OR = 1.55, 95 % CI [1.28, 1.88], P = 0.000; 4G/4G + 5G/4G versus 5G/5G (dominant model): OR = 1.56, 95 % CI [1.19, 2.03], P = 0.001; 4G/4G versus 5G/4G + 5G/5G (recessive): OR = 1.90, 95 % CI [1.46, 2.48], P = 0.000; 4G/4G versus 5G/5G (homozygote model): OR = 2.24, 95 % CI [1.68, 2.98], P = 0.000); 4G/4G + 5G/5G versus 5G/4G: OR = 1.26, 95 % CI [1.01, 1.58], P = 0.040. No significant association was found in heterozygote model (5G/4G versus 5G/5G: OR = 1.22, 95 % CI [0.94, 1.57], P = 0.133), consistently (ESM 2, Table). Subgroup analysis based on thrombus type found no significant association in any of the genetic models tested.
Sensitivity analysis was carried out on the six genetic model. Heterogeneity was reduced when one study [12] was removed. Results showed that the pooled OR estimate was similar with before (ESM 3, Table).
Begg’s funnel plot and Egger’s test were performed to assess the publication bias of included studies. There was no statistical evidence of publication bias in our meta-analysis (ESM 1, Table).
Discussion
Patients with thrombotic mainly have functional imbalance between coagulation system and fibrinolytic system, which is often attributed to high-level expression of PAI-1 [31]. PAI is the most important component in fibrinolytic system, which accounts for about 60 % of the activity of inhibitors. PAI -1 play an important role in the regulation of fibrinolysis, therefore, its overexpression may promote thrombosis of events. PAI-1 4G/ 5G polymorphism is related to the level of PAI-1 in plasma. Several studies have investigated the relationship between PAI-1 4G/5G polymorphism and the risk of venous thrombosis [10–13, 22, 31]. However, the results were controversial. The results of individual case-control studies may produce contradictory results due to sample selection, race and region, etc., and the association between genetic polymorphism and disease susceptibility cannot be objectively judged. Therefore, we conducted an updated meta-analysis on the relationship between PAI-1 4G/5G gene polymorphism and VTE risk.
In recent years, with the development of the evidence medical science, meta-analysis which improves the statistical efficiency of the original results and solves the inconsistencies among individual results by integrating the results of multiple samples of the same topic and increasing the sample size, is increasingly applied in the medical field. In this meta-analysis, data from 27 case-control studies were combined to evaluate the association between PAI-1 4G/5G polymorphism and VTE risk, and the results suggested that PAI-1 4G/5G polymorphism was associated with the risk of VTE. Therefore, PAI-1 4G/5G genotyping can be a risk factor for high risk population for VTE. Subgroup analysis based on ethnicity revealed significantly increased risk in Asians, which is consistent with the previous meta-analysis [34]. In addition, Wang [34] also found that the PAI-1 4G/5G polymorphism was related to the risk of venous thromboembolism in Caucasians, but no significant association was found in this study. Subgroup analysis based on thrombus type found that PAI-1 4G/5G polymorphism was also associated with increased risk of DVT in Wang’s [34] analysis, but no significant association in any of the genetic models tested in this study. On the contrary, PAI-1 4G/5G polymorphism was related to the risk of venous thromboembolism in Asia, but not related to the type of thrombosis in this study. Such discrepancies may due to that the data in Wang’s [34] analysis did not reach HWE can increase the chance of a false-positive association.
In this study, Begg’s funnel plot and Egger’s test were performed to assess the publication bias in six genetic models of included studies, and the results showed that there was no publication bias in the included studies, which indicates that the relevant literature collected in this study is comprehensive and there is no publication bias.
Heterogeneity deserves to pay more attention in meta-analysis, and one of the most important objectives of meta-analysis is to determine the sources of heterogeneity. Heterogeneity exists in all six genetic models in this study. Exclusion of one study at a time was performed to eliminate heterogeneity. Heterogeneity was reduced when Farajzadeh’s study [12] was removed. It is possible that the research objects in this document were Iranian. In ethnicity subgroup analysis, the heterogeneity (I2) is 27.90 %~60.30 % in Asians and 60.60 %~87.10 % in Caucasian, showing that the genetic background factors of different races are also the main reasons of heterogeneity. In addition, sensitivity analysis showed that the combined effects of each genetic model were similar. Therefore, the results of this study are reliable.
Limitations
There are some limitations in this study: (1) only Chinese and English literature are included, which may lead to insufficient data coverage of other ethnic; (2) only Asian and Caucasian data were provided in the included literature of this study, genotype data of other races, such as African, were not available, which reduced the comprehensiveness of the results. (3) subgroup analysis and sensitivity analysis only explained a part of heterogeneity, but heterogeneity still existed.
Conclusions
In summary, though with limitations, our meta-analysis suggested that PAI-1 4G/5G polymorphism is associated with the risk of venous thromboembolism, especially in Asians. Larger studies from multi-ethnic populations are needed to further clarify the association between PAI-1 4G/5G polymorphism and VTE risk.
Electronic supplementary material
The electronic supplementary material (ESM) is available with the online version of the article https://doi.org/10.1024/0301-1526/a000839
References
1 “Post-thrombotic panic syndrome”: A thematic analysis of the experience of venous thromboembolism. Br J Health Psychol. 2016;22:8–25.
2 A genetic risk score comprising known venous thromboembolism loci is associated with chronic venous disease in a multi-ethnic cohort. Thromb Res. 2015;136:966–73.
3 Plasminogen activator inhibitor 1: physiological and pathophysiological roles. News Physiol Sci. 2002;17:56–61.
4 . Inherited and acquired risk factors for venous thromboembolism. Semin Respir Crit Care Med. 2012;33:127–37.
5 Plasminogen activator inhibitor-1 (PAI-1) gene 4G/5G promoter polymorphism is seen in higher frequency in the Indian patients with deep vein thrombosis. Clin Appl Thromb Hemost. 2010;16:184–8.
6 . Effect of plasminogen activator inhibitor-1 4G/5G polymorphism in Turkish deep vein thromboembolic patients with and without prothrombin 20210 G-A. Turk J Haematol. 2004;21:83–6.
7 Prospective study of fibrinolytic markers and venous thromboembolism. J Clin Epidemiol. 2003;56:598–603.
8 Association of deep venous thrombosis with prothrombotic gene polymorphism identified in lung cancer cases. Mol Biol Rep. 2011;38:2395–400.
9 Genetic mutations in Turkish population with pulmonary embolism and deep venous thrombosis. Clin Appl Thromb Hemost. 2011;17:E87.
10 Is genetic screening necessary for determining the possibility of venous thromboembolism in cancer patients? Med Princ Pract. 2012;21:160–3.
11 . Plasminogen activator inhibitor-1 gene 4G/5G polymorphism in cancer patients with and without thrombosis. J Thromb Thrombolysis. 2006;22:111–2.
12 Polymorphisms in thrombophilic genes are associated with deep venous thromboembolism in an Iranian population. Meta Gene. 2014;2:505–13.
13 The association between the 4G/5G polymorphism in the promoter of the plasminogen activator inhibitor-1 gene and extension of postsurgical calf vein thrombosis. Blood Coagul Fibrinolysis. 2013;24:237–42.
14 Vascular involvement in Behcet’s disease: Relation with thrombophilic factors, coagulation activation, and thrombomodulin. Am J Med. 2002;112:37–43.
15 A novel G/A and the 4G/5G polymorphism within the promoter of the plasminogen activator inhibitor-1 gene in patients with deep vein thrombosis. Thromb Res. 1996;84:431–43.
16 The investigation of angiotensin converting enzyme I/D and plasminogen activator inhibitor-1 4G/5G polymorphisms in venous thromboembolism patients. Tuberk Toraks. 2013;61:88–95.
17 No evidence for plasminogen activator inhibitor 1 4G/4G genotype as risk factor for cerebral venous thrombosis. J Neurol. 2007;254:1124–5.
18 Major and potential prothrombotic genotypes in patients with venous thrombosis and in healthy subjects from Slovenia. Pathophysiol Haemost Thromb. 2008;36:58–63.
19 Do thrombophilic gene mutations have a role on thromboembolic events in cancer patients? Asia-Pac J Clin Onco. 2012;8:e34–41.
20 Factor V Leiden, prothrombin 20210G –> A, methylenetetrahydrofolate reductase 677C –> T and plasminogen activator inhibitor 4G/5G polymorphism in women with pregnancy-related venous thromboembolism. Eur J Obstet Gynecol Reprod Biol. 2003;111:157–63.
21 The role of plasminogen activator inhibitor-1 polymorphism, factor-V-Leiden, and prothrombin-20210 mutations in pulmonary thromboembolism. Clin Appl Thromb Hemost. 2009;15:73–7.
22 . Investigation of Plasminogen Activator Inhibitor-1 (PAI-1) 4G/5G promoter polymorphism in Indian venous thrombosis patients: A case-control study. Eur J Haematol. 2017;99:249–54.
23 Promotor polymorphisms of plasminogen activator inhibitor-1 and other thrombophilic genotypes in cerebral venous thrombosis: a case-control study in adults. J Neurol. 2012;259:2287–92.
24 Genetic polymorphisms in venous thrombosis and pulmonary embolism after total hip arthroplasty: a pilot study. Clin Orthop Relat Res. 2009;467:1507–15.
25 Comparison of the plasminogen activator inhibitor-1 4G/5G gene polymorphism in females with venous thromboembolism during pregnancy or spontaneous abortion. Clin Hemorheol Microcirc. 2008;39:329–32.
26 The 4G/5G polymorphism of the type 1 plasminogen activator inhibitor gene and thrombosis in patients with antiphospholipid syndrome. Arthritis Rheum. 2000;43:2349–58.
27 Influence of the -675 4G/5G dimorphism of the plasminogen activator inhibitor 1 promoter on thrombotic risk in patients with factor V Leiden. Br J Haematol. 2000;110:135–8.
28 A study on the relationship of plasminogen inhibitor-1 (PAI-1) and its polymorphisms of 4G/5G in promoter region with deep venous thrombosis after operation. J Guiyang Med Coll. 2005;30:10–4.
29 Association of 4G/5G polymorphism in PAI1 promoter with PAI1 level in deep vein thrombosis. Chin J Med Genet. 2005;22:624–7.
30 Association between PAI-1 activity and its polymorphism in Uygur patients with venous thromboembolism of Xinjiang. J Clin Cardiol. 2014;30:576–82.
31 Association between plassinogen activator inhibitor-1 promoter gene polymorphism and venous thromboembolism. Pharm Clin Res. 2018;26:261–4.
32 Study on the relationship of pulmonary thromboembolism with tissue plasminogen activator and its inhibitor. Chinese Chin J Geriatr Heart Brain Ves Dis. 2010;12:967–9.
33 Relationship between polymorphisms of plasminogen activator inhibitor-1 promoter gene and pulmonary thromboembolism in Chinese Han population. Natl Med J China. 2006;86:1313–7.
34 Association between the plasminogen activator inhibitor-1 4G/5G polymorphism and risk of venous thromboembolism: a meta-analysis. Thromb Res. 2014;134:1241–8.



