Cancer, cancer treatment and aneurysmatic ascending aorta growth within a retrospective single center study
Abstract
Summary:Background: Multi-morbidity poses a substantial challenge for health care in an aging population. Recent studies did not provide evidence for general side effects of anti-cancer therapy regarding the growth rate of coincident abdominal aortic aneurysms, although it was suggested that specific therapeutic substances might accelerate growth. Aneurysm pathology, however, differs with respect to localization. Hence, we present the first ever analysis on the association of cancer and cancer therapy with growth alteration of aneurysms of the ascending aorta (AscAA). Patients and methods: A retrospective single-center identification of AscAA+cancer patients was performed in the institutional picture archiving and communication system (PACS). Included were all patients with ≥2 CT angiograms over ≥6 months and additional malignancy. Clinical data and aneurysm diameters were retrieved and analyzed for an association of cancer (stratified by tumor entity) or cancer therapy (stratified by several classes of chemotherapeutic agents and radiation therapy) with annual growth rate, respectively. Statistics included t-test, Wilcoxon test, and a linear regression model accounting for initial AscAA diameter and type of treatment. Results: From 2003 to 2021, 151 patients (median age 70 years; 85% male) with AscAA and coincident 163 malignancies were identified. Prostate (37%) and hematologic cancer (17%) were most frequent. One-hundred-eleven patients (74%) received chemotherapy and 75 patients (50%) had radiation. After exclusion of six patients with an initial AscAA diameter >55 mm, the average annual AscAA growth rate was 0.18±0.64 mm/year, with only 12 patients experiencing a growth rate >1mm/year. Neither tumor entity nor radiation or chemotherapy – alone or in combination – were significantly associated with an alteration of the annual AscAA growth rate. Likewise, a subanalysis for singular chemotherapeutic agents did not reveal a specific association with AscAA growth alteration. Conclusions: Growth rates of AscAA are low in this cohort with coincident malignancy. Cancer and/or chemotherapy or radiation are not associated with an alteration of the annual growth rate. Additional control examinations seem unnecessary.
Introduction
An aneurysm of the ascending aorta (AscAA) is a rare entity of aneurysmal degeneration, yet accounts for approx. 50% of all thoracic aortic aneurysms including pathologies of the aortic arch and the descending thoracic aorta with a combined estimated incidence of approx. 10/100,000 [1, 2]. The imminent threat is rupture – and in the case of AscAA – aortic insufficiency. Hence, guidelines recommend AscAA repair based on a transverse diameter threshold around 45–55 mm accounting for etiology, aortic valve insufficiency, and aneurysm growth rate [3, 4].
The etiology of AscAA remains often unclear. The underlying pathophysiology is medial degeneration with a consecutive loss of elastic properties of the conduit and biological remodeling of the aneurysm wall [5, 6, 7]. Many singular genetic diseases such as Marfan-, Ehlers-Danlos- or Loeys-Dietz-syndrome, familial thoracic aortic aneurysm and dissection (TAAD) or congenital conditions with unclear genetics, such as bicuspid aortic valve (BAV), are clearly associated with AscAA [2, 3, 4]. Additionally, autoimmune disease and (post-infective) aortitis might be of importance, but a high rate of normal degenerative tissue specimen was observed after aortic replacement [5].
The co-prevalence of malignant disease and AscAA is unknown, however, co-incidentally discovered cardiovascular and malignant diseases upon modern imaging are increasing [8, 9]. For example, approximately 5% of patients with abdominal aortic aneurysm (AAA), the most frequent aneurysm entity, are estimated to suffer from an additional malignancy simultaneously and might thus be considered for therapy and/or monitoring of both pathologies [10]. Previous publications have suggested, though not proven altered aneurysm growth rates as effect of different chemotherapeutics on e.g., AAA. Yet, the evolving spectrum of therapies might be associated with a differential effect on aneurysm growth [11, 12].
No such analysis has been conducted in a patient cohort with AscAA and cancer [13]. Thus, we hypothesized that modern chemotherapeutic regimen might influence the growth of pre-existing AscAA and, therefore, performed a retrospective analysis of annual aneurysm growth rates in a patient cohort with both conditions.
Patients and methods
Patient identification and inclusion criteria
Patients were retrospectively identified in the institutional PACS database. All CT examination reports from thorax, abdomen and/or pelvis examinations acquired at the study center between January 1st, 2003 and February 28th 2021, were screened using a full-text query including the key words “aneurysm” and “carcinoma”, “tumor”, “chemotherapy” and the different types of carcinomas (Figure 1 and electronic supplementary material [ESM] 1).
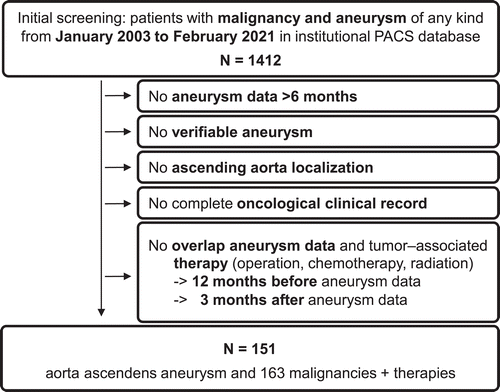
Patient data were pseudonymized for further analysis. The study was performed in accordance with the declaration of Helsinki and approved by the local ethics committee (Ethikkommission Klinikum rechts der Isar: 195/20S). The manuscript is designed following the STROBE statement [14].
Inclusion/exclusion criteria
Inclusion criteria were presence of a malignant disease and presence of an ascending aortic aneurysm (transverse diameter >40 mm), confirmed by CT or CT angiography (CTA) including PET/CT. In addition, for longitudinal aneurysm growth, a CT-based follow-up period of ≥6 months covering ≥2 individual CT(A)-examinations was required.
Tumor diagnosis and therapy (no therapy, chemotherapy, radiation, operation and combinations thereof) had to overlap with the diagnosis of any aneurysm, and patients were included, if: (i) an ongoing therapy started 12 months before the baseline CT; (ii) therapy was within the CT-observation period; (iii) therapy was initiated >3 months before the last CT(A).
Patients with aneurysm diameters exceeding the threshold for operation upon detection who did not receive any kind of aneurysm treatment are shown in the cohort overview, but were excluded from any growth analysis due to missing data on natural growth. Patients who received any kind of aneurysm repair (open or endovascular) were not followed-up after surgery. Ascending Aorta was defined covering the aortic root with the sinuses of Valsalva, the sinotubular junction and tubular ascending aorta extending to the aortic arch (innominate artery) [2, 3].
Exclusion criteria were age <18 years, incomplete clinical (no established tumor diagnosis, missing information regarding cancer therapy) or imaging data, or missing temporal association of tumor and/or therapy with aneurysm (see above). Patients with only aneurysms other than AscAA (e.g., cerebral aneurysms) were excluded and are reported elsewhere [15, 16]. Aneurysms including the aortic arch were excluded.
Outcome parameters
The primary outcome was the average annual aneurysm growth during the study period. The secondary outcome was the average annual aneurysm growth stratified for the different oncological treatments (chemotherapy/radiation), type of cancer and specific chemotherapeutic substances. Tertiary outcomes included the aneurysm growth under different types of chemotherapeutic agents stratified for the initially measured aortic diameter.
Aneurysm growth
All patients with an initial diameter of the ascending aorta of at least 35 mm, who reached at least 40 mm during the individual observation period were included. The annual AscAA growth rate was calculated based on the “first-last diameter/time” method as reviewed before [17]. For patients with an observation period between six months and one year we calculated the annual AscAA growth rate to compare those cases to the literature and the other patients.
Based on a thorough literature search, an annual growth rate of ≤1mm/year was considered normal [18, 19]. Due to a lack of data, this growth rate was considered independent of the initial diameter [2, 19]. Although the inter-observer variability is high and the diameter depends on the cardiac cycle in ascending aortic diameter measurements, a 1 mm difference was considered to be real within the same patient [20, 21, 22].
Stepwise analysis process
The identified patients that met the inclusion criteria were individually assessed based on electronic patient files and medical records by two reviewers (ABR, KK) independently. All CT examinations had been performed after IV application of contrast agent, but examinations with different timings were used for this analysis: Routine CT staging examinations had been acquired in the portal venous phase, while examinations termed CT angiography (CTA) had been acquired in the arterial phase. Since there is a visible limitation between the intraluminal volume and the aortic wall due to the contrast medium, it remains feasible to precisely measure the diameter of large vessels such as the ascending aorta in both phases. In every CT(A) the aneurysm reported in the examination report was reviewed and a centerline-based leading edge transversal measurement of the maximum diameter was obtained by three independent reviewers (KK, BS, AB) using a 3D multiplanar reconstruction (MPR), while two were completely blinded to the medical records (BS, AB). Consensus was reached upon discrepancy by joint re-assessment. Most examinations were not electrocardiographic (ECG) gated (>90%). No CT(A)s from outside the study center were analyzed.
In case of any significant AscAA growth (s. above), if not documented in the medical records, the shape of the aortic valve (bicuspid/tricuspid) was attempted to be assessed in the CT(A)s.
The patient baseline characteristics included sex, age, comorbidities (arterial hypertension, coronary artery disease, hyperlipidemia, diabetes, peripheral artery disease and smoking status) and co-medication (antiplatelets, statins, antihypertensives, metformin, insulin; Table I). Particularly, the presence of any connective tissue disease (CTD) was investigated in the medical record. Specific malignancies were grouped based on affected organ and prevailing malignancy based on the International Classification of Diseases (ICD; ESM 1). Tumor stages were summarized by the TNM staging system. Specific therapies were grouped based on their respective mode of action (ESM 2). Individual cycles and different regimen of chemotherapy were assessed separately but not included for further analysis. Radiation therapy (radiation plans unavailable) and tumor operations were not further stratified.
Statistical analysis
Where applicable, average values are shown as median (interquartile range: IQR) or average±standard deviation (SD). All statistical analyses were performed using R version 4.0.3, and graphics were created using the ggplot2 package. The level of significance was set at p<0.05. For the comparison of two groups (chemo YES/NO, radiation YES/NO, type of cancer YES/NO, individual classes of chemotherapeutic agents YES/NO), both a Student’s t-test and a Wilcoxon test was used (Figure 2B, ESM 5 and ESM 7).
For the association of certain therapies with aneurysm growth, a linear regression model with the start diameter as confounder was used: (“rate=est.×therapy+a×dmax0+intercept”; est., a, dmax0=regression coefficients). The value of interest is the estimated correlation (est.) of the binary variable “therapy YES/NO” with the aneurysm growth rate. The outcome of this parameter is given as difference in growth rate between the two groups (YES/NO; ESM 6).
Results
During an 18-year period, 151 patients with concomitant malignancy and AscAA met the inclusion criteria (Figure 1). Thereof, 85.4% were male and the median age was 68.8 years (IQR: 63.8–74.6). Comorbidities, cardio-vascular risk profile and patient details are shown in Table I. In seven patients one additional aneurysm each was discovered (3× iliac, 3× AAA, 1× celiac trunk – all without indication for surgical repair). Average observation time per patient was 3.9±3.2 years.
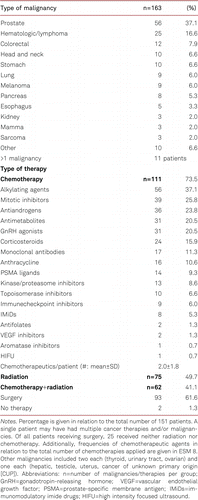
The 151 patients had a total of 163 malignancies. Prostate cancer (37.1%) and hematologic malignancies/lymphoma (16.6%) were seen most frequently (Table II). The majority of patients (n=111, 73.5%) received chemotherapy either alone or in combination with radiation and/or surgery (Table II). Seventy-five patients (49.7%) had radiation either alone or in combination. Six patients (4.0%) were initially diagnosed with an aneurysm exceeding the surgical threshold of 55 mm and were not operated due to e.g., inoperability/missing consent (ESM 3).
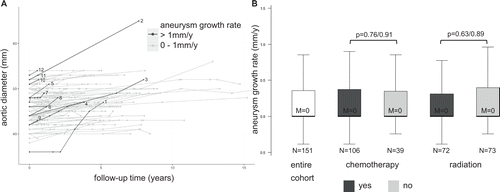
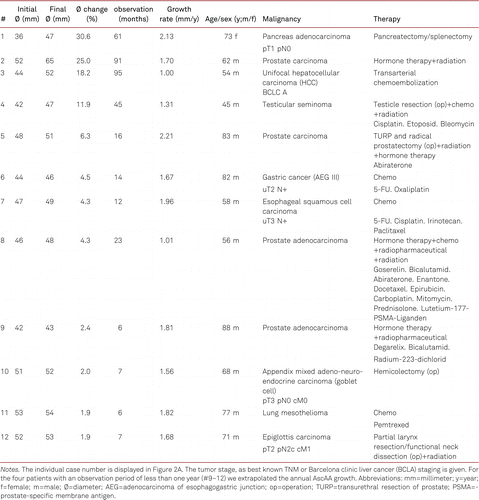
In the remaining 145 patients, the average initial AscAA diameter was 46.01±3.74 mm and the average final diameter 46.97±4.07 mm, with an average aneurysm growth rate of 0.18±0.64 mm/y (ESM 4). Of these patients, 12 (8.3%) had a growth rate exceeding 1 mm/year (1.66±0.39 mm/y) with no BAV or CTD diagnosed (Figure 2A, Table III, ESM 4). Three patients (2.1%) were operated on the aortic valve or ascending aorta during the observation time. No aneurysm- or surgery-related death and no aneurysm rupture occurred.
Oncologic therapy, malignancy and ascending aneurysm growth
Patients receiving chemotherapy alone or in combination did not show any significantly different growth rates compared to patients without chemotherapy (chemo YES/NO: 0.17±0.65 vs. 0.21±0.65 mm/y, p=0.76; Figure 2B). The same was true for patients receiving radiotherapy alone or in combination compared to patients without radiotherapy (radio YES/NO: 0.16±0.52 vs. 0.21±0.75 mm/y, p=0.63; Figure 2B). No singular chemotherapeutic administered to at least five patients was significantly associated with altered AscAA growth rates, even when stratified for initial diameter (ESM 5 and 6). Also, no specific type of malignancy was associated with a significantly altered growth rate (ESM 7).
The 12 patients presented here with a considerable growth rate (>1mm/y) received a heterogeneous plethora of treatments for their respective malignancy (Table III).
Discussion
This study is to our best knowledge the first analysis of AscAA growth rates in a patient cohort with coincident malignancy and demonstrates that cancer and anti-cancer therapy are not associated with altered growth rates in this cohort (Figure 2).
Heart and ascending aorta have the same embryologic origin and cardiotoxicity is a common adverse effect of chemotherapy [23, 24]. For example, anthracyclines and other classic chemotherapeutic agents may cause heart failure, while novel agents such as immune checkpoint inhibitors are associated with myocarditis [25, 26]. The aneurysm wall is a biologically active tissue and, thus, systemic effects of chemotherapeutic agents on aneurysm growth cannot be excluded. There are multiple case reports of aneurysm rupture or rapid growth in cancer patients [27, 28]. Previous publications have speculated that the abdominal aorta might be more prone to chemotherapy-associated off target effects [11, 12]. In a retrospective cohort study of AAA patients with concomitant malignancy, we could demonstrate that radiotherapy was associated with a significantly lower growth rate and that specific chemotherapeutic substances e.g., antimetabolites were associated with a significantly accelerated annual AAA growth rate, although chemotherapy in general was not associated with significantly altered aneurysm growth [16]. However, growth rates are different for AAA compared to AscAA, and the pathophysiology might differ due to an unrelated embryologic development [2, 17, 29, 30, 31, 32].
Due to the slow growth rate of AscAA, the diversity of tumor entities and anti-cancer medication, and the constant advances in the field of oncology (first-in-class medications etc.) a larger cohort size and a longer observation interval (preferably in a prospective registry) might be necessary to identify potential singular effects on AscAA growth (Figure 2, ESM 5 and 7). Similarly, other parameters such as aneurysm volume, valve function or ECG-gated based diameter could lead to more accurate measurements in future studies [33].
The median age of the presented cohort was 70 years, and the typical cardiovascular risk profile that is also seen in similar cohorts undergoing AscAA surgery was observed (Table I) [34, 35]. Hence, in the case of aneurysm growth and eventual exceedance of the diameter threshold, treatment, either open or endovascular, should not be deferred in such patients [36, 37]. Currently, annual or semi-annual CT or MRI diagnostics is recommended for follow-up of the AscAA depending on maximum diameter [2, 4]. The data presented here does not support a different scheme for patients with co-incident cancer that may require chemotherapy (Figure 2).
Limitations
Our study is limited by the retrospective nature, the relative scarcity of the co-incidence of both conditions, and the heterogeneity of malignancies and therapies, which usually have even been applied in combination (ESM 1 and 2). Furthermore, there may have been a registration bias that resulted in the exclusion of patients with AscAA requiring intensive monitoring or even discontinuation of cancer therapy. CT documentation from outside the study center was not available and, thus, not analyzed. Consequentially, some included patients may lack intermediate data points or patients may have been lost to follow-up. Due to a low number of female patients included in the study (14.6%), the results presented here cannot be generalized to the female population. Unfortunately, our study is missing an appropriate control group of AscAA patients without malignancy for comparison. Moreover, control group data could not be retrieved from the literature.
For patients with an observation period of less than one year we extrapolated the annual AscAA growth rate to compare those cases to the literature and the other patients. Additionally, the low number of ECG-gated CTs might render the diameter measurements and the calculated growth rates less accurate [38]. ECG-gating is usually only performed in the case of dissections or cardiac pathologies, and not for cancer CT staging. Estimates of the average diameter difference for the ascending aorta between systole and diastole vary throughout the literature and are in the range of 7% or ~1.5 mm, respectively [22, 39]. However, non-ECG measurements have been found to be consistent with ECG measurements [39]. All measurements were done by two experienced vascular surgeons and supervised by an experienced radiologist to minimize inter-observer variability [40, 41]. Lastly, the frequency of individual chemotherapeutics was low, which impeded statistical analysis and may have led to type I errors in subgroups with very few patients (ESM 5).
Conclusions
Growth rates of the aneurysmatic ascending aorta are generally low in this study cohort with AscAA and concomitant malignancy. Cancer and cancer therapy are not associated with significant growth alterations here. Hence, a modification of the current follow-up might not be necessary.
Electronic supplementary material
The electronic supplementary material (ESM) is available with the online version of the article at https://doi.org/10.1024/0301-1526/a001038
References
1 . Epidemiology of aortic aneurysm in the United States. J Clin Epidemiol. 1995;48(11):1289–98.
2 . The ascending aortic aneurysm: When to intervene? IJC Heart Vasc. 2015;6:91–100.
3 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases. Kardiol Pol. 2014;72(12):1169–252.
4 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Anesth Analg. 2010;111(2):279–315.
5 Surgical pathology of the ascending aorta: a clinicopathologic study of 513 cases. Am J Surg Pathol. 2006;30(9):1159–68.
6 Hyperplastic cellular remodeling of the media in ascending thoracic aortic aneurysms. Circulation. 2005;112(8):1098–105.
7 Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J Thorac Cardiovasc Surg. 2006;131(3):671–8.
8 Global multimorbidity patterns: a cross-sectional, population-based, multi-country study. J Gerontol A Biol Sci Med Sci. 2016;71(2):205–14.
9 . Trends of multimorbidity in 15 European countries: a population-based study in community-dwelling adults aged 50 and over. BMC Public Health. 2021;21(1):76.
10 Current surgical management of abdominal aortic aneurysm with concomitant malignancy in the endovascular era. Surg Today. 2016;46(8):985–94.
11 . Aortic aneurysm natural progression is not influenced by concomitant malignancy and chemotherapy. Ann Vasc Surg. 2021;71:29–39.
12 . The effect of chemotherapy for malignancy on the natural history of aortic aneurysm. J Vasc Surg. 2015;61(1):50–7.
13 Immunosuppressive agents and thoracic aortic aneurysm: real correlation or mere coincidence? Thorac Cardiovasc Surg. 2021. Online ahead of print.
14 The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7.
15 Concomitantly discovered visceral artery aneurysms do rarely grow during cancer therapy. Clin Anat. 2021. Online ahead of print.
16 Radiation and chemotherapy are associated with altered aortic aneurysm growth in patients with cancer: impact of synchronous cancer and aortic aneurysm. Eur J Vasc Endovasc Surg. 2022;64(2–3):255–64.
17 . Systematic review and meta-analysis of growth rates of small abdominal aortic aneurysms. Br J Surg. 2011;98(5):609–18.
18 . Procedures for estimating growth rates in thoracic aortic aneurysms. J Clin Epidemiol. 1998;51(9):747–54.
19 Association of mortality and acute aortic events with ascending aortic aneurysm: a systematic review and meta-analysis. JAMA Netw Open. 2018;1(4):e181281.
20 Fully automated guideline-compliant diameter measurements of the thoracic aorta on ECG-gated CT angiography using deep learning. Quant Imaging Med Surg. 2021;11(10):4245–57.
21 Dynamism of the aortic annulus: Effect of diastolic versus systolic CT annular measurements on device selection in transcatheter aortic valve replacement (TAVR). J Cardiovasc Comput Tomogr. 2016;10(1):37–43.
22 . Ascending aorta measurements as assessed by ECG-gated multi-detector computed tomography: a pilot study to establish normative values for transcatheter therapies. Eur Radiol. 2009;19(3):664–9.
23 .
Bildung der Herzschleife . In: Aumüller GAust GConrad AEngele JKirsch JMaio G, Hrsg. Duale Reihe Anatomie. 5. korrigierte Auflage. Georg Thieme Verlag; 2020.24 Cardiotoxic effects of chemotherapy: A review of both cytotoxic and molecular targeted oncology therapies and their effect on the cardiovascular system. Crit Rev Oncol Hematol. 2018;126:186–200.
25 . Heart failure in relation to anthracyclines and other chemotherapies. Methodist Debakey Cardiovasc J. 2019;15(4):243–9.
26 . Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. 2020;9(2):e013757.
27 . Acute enlargement and subsequent rupture of an abdominal aortic aneurysm in a patient receiving chemotherapy for pancreatic carcinoma. J Vasc Surg. 2000;32(1):197–200.
28 . Effects of chemotherapy in patients with concomitant aortic aneurysm and malignant disease. Ann Vasc Surg. 2017;45:268.e13–268.e20.
29 . Regional heterogeneity within the aorta: relevance to aneurysm disease. J Thorac Cardiovasc Surg. 2008;136(5):1123–30.
30 . Literature review of surgical management of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2001;22(3):197–204.
31 Growth rate of ascending thoracic aortic aneurysms in a non-referral-based population. J Cardiothorac Surg. 2022;17(1):14.
32 Systematic review of the growth rates and influencing factors in thoracic aortic aneurysms. Eur J Vasc Endovasc Surg. 2016;51(5):674–81.
33 . Imaging of the aortic root on high-pitch non-gated and ECG-gated CT: awareness is the key! Insights. Imaging. 2020;11(1):51.
34 . Endovascular treatment of non-dissected ascending aorta disease: a systematic review. Eur J Cardiothorac Surg. 2018;53(2):317–24.
35 Is the Bentall procedure for ascending aorta or aortic valve replacement the best approach for long-term event-free survival? Ann Thorac Surg. 2003;76(3):698–703. Discussion 703.
36 . Replacing the ascending aorta in the elderly: do or do not. Indian J Thorac Cardiovasc Surg. 2019;35(Suppl 2):106–11.
37 Elective surgery for ascending aortic aneurysm in the elderly: should there be an age cut-off? Eur J Cardiothorac Surg. 2017;51(5):965–70.
38 . Thoracic aorta: motion artifact reduction with retrospective and prospective electrocardiography-assisted multi-detector row CT. Radiology. 2002;222(1):271–7.
39 . Comparison of dynamic changes in aortic diameter during the cardiac cycle measured by computed tomography angiography and transthoracic echocardiography. J Vasc Surg. 2019;69(5):1538–44.
40 Intra- and interobserver variability in the measurements of abdominal aortic and common iliac artery diameter with computed tomography. The Tromso study. Eur J Vasc Endovasc Surg. 2003;25(5):399–407.
41 A novel software tool for semi-automatic quantification of thoracic aorta dilatation on baseline and follow-up computed tomography angiography. Int J Cardiovasc Imaging. 2019;35(4):711–23.



