Emotional Reactivity, Emotion Regulation, and Social Emotions in Affective Disorders
Neural Models Informing Treatment Approaches
Abstract
Abstract. Affective disorders, specifically Major Depressive Disorder and Bipolar Disorders, show high prevalence, relapse rates, and a high likelihood to develop a chronic course. For the past two decades, research has investigated the neural correlates of emotion processing and emotion regulation in patients with affective disorders. Putative underlying causal mechanisms of dysregulated affect have been informed by knowledge from the intersection of neuroimaging and clinical psychology. More recent investigations also consider processing the role of mostly negative, self-blaming social emotions, which have been linked to treatment resistance and, hence, provide a prolific target for intervention. Several psychotherapeutic treatment approaches already focus on emotion, and here specific knowledge about the mechanisms underlying persistent changes in affect bears the potential to improve the treatment of affective disorders. In this narrative review, we delineate why and how our insights into the neural correlates of emotion processing and regulation can be applied to the treatment of patients with affective disorders.
Behandlungsansätze
Zusammenfassung. Affektive Störungen, insbesondere die Major Depression und bipolare Störungen, weisen eine hohe Prävalenz, häufige Rückfälle und eine hohe Rate an chronischen Krankheitsverläufen auf. In den letzten zwei Jahrzehnten hat die Forschung die neuronalen Korrelate der Emotionsverarbeitung und -regulation bei Patient_innen mit affektiven Störungen untersucht. Die mutmaßlichen Mechanismen der gestörten Affektregulation wurden durch Erkenntnisse aus der biologischen und klinischen Psychologie untermauert. Neuere Untersuchungen befassen sich auch mit selbstbeschuldigenden sozialen Emotionen, die mit Behandlungsresistenz in Verbindung gebracht werden und daher ein ergiebiges Ziel für Interventionen darstellen. Psychotherapeutische Behandlungsansätze konzentrieren sich bereits auf die emotionale Verarbeitung, jedoch birgt hier spezifisches Wissen über die Mechanismen, die anhaltenden affektiven Veränderungen zugrunde liegen, das Potenzial, die Behandlung von affektiven Störungen zu verbessern. In dieser narrativen Übersichtsarbeit wird dargelegt, warum und wie unsere Erkenntnisse über die neuronalen Korrelate der Emotionsverarbeitung und -regulation bei der Behandlung von Patient_innen mit affektiven Störungen eingesetzt werden können.
Affective disorders contribute substantially to disability worldwide (Murray et al., 2012). Both Major Depressive Disorder (MDD) and Bipolar Disorders (BD) entail high direct and indirect economic costs (Murray et al., 2012). Despite existing treatment approaches that are effective at least in the short term, more than 50 % of patients with affective disorders experience relapse (Gitlin et al., 1995; Sim et al., 2015). The hallmark symptoms of BD patients are mood states alternating between (hypo–)mania and depression, while patients with MDD experience exclusively depressive episodes. It has long been suggested that these pathological affective states may be caused by altered emotional reactivity (Phillips et al., 2003), and the extensive knowledge about the neurobehavioral dynamics of emotion processing in affective disorders gained over the last two decades may inform and improve clinical practice. Given the comparatively high cost that MRI research entails, it is important to understand why neuroimaging studies are indispensable for understanding emotional processes. First, neural changes provide additional insight into changes in psychological processes which are not apparent from assessing the experiential and behavioral levels alone. For example, there is evidence of altered neural activity in patients during explicit emotion regulation, even though no alterations are observable on the behavioral level in patients with BD (Kurtz et al., 2021). Simply put, this may mean that patients need to use more resources to regulate emotions. Second, neural correlates provide additional information on emotion processing which, unlike self-assessments and behavioral measures, cannot be directly and volitionally influenced by the patient. For example, if we survey how strongly participants react emotionally in self-report, some people may have difficulty identifying or describing their own emotions – a characteristic also described as alexithymia (Sifneos, 1973). Importantly, alexithymia is frequently observed in patients with affective disorders, adding another source of noise to investigations of emotion in patients and control participants relying on self-report (Förster et al., 2020; Herold et al., 2017; Luminet et al., 2001; Ospina et al., 2019). Here, neural activation in emotion-processing circuits may provide additional information that does not depend on patient introspection. Consequently, neuroimaging studies allow for a more comprehensive examination of emotion-processing behavioral and self-report studies alone cannot provide.
In this narrative review, we summarize current knowledge stemming from clinical neuroscience, clinical-psychological research, and psychotherapeutic practice to draw conclusions for targeted intervention.
While many findings concerning neural and behavioral changes in emotion processing and regulation in affective disorders have been accumulated over the past two decades and summarized in large and widely cited review papers (e. g., Phillips et al., 2003; Stuhrmann et al., 2011; Townsend & Altshuler, 2012), changes in social emotions in affective disorders have received little attention (for a review in patients with major depressive disorder, see Kupferberg et al., 2016). Therefore, in this review, we seek to combine the extensive knowledge of emotion processing in affective disorders based on previous review articles with emerging evidence from studies of social emotions in affective disorders to provide a more comprehensive picture of potential mechanisms that can be targeted with treatments.
First, we provide an overview of the neural correlates associated with emotional reactivity and emotion regulation in affective disorders. We further report on alterations of social emotions, which have been associated with an increased vulnerability to becoming depressed as well as a lower treatment response during depression (Rector et al., 2000; Kim et al., 2011). Finally, we report on two treatment approaches based on neuroscientific insights specifically into emotion regulation and social emotions: emotion-regulation therapy (ERT) and compassion-focused therapy (CFT).
Emotional Reactivity
Emotional reactivity refers to the initial, relatively spontaneously developing emotional response to internal or external stimuli. Strong emotional reactivity could then be understood as the tendency to experience frequent and intense emotional arousal. Both the threshold and ease with which individuals become emotionally aroused and the intensity of emotional experiences are aspects of emotional reactivity. Although emotional reactivity is often thought of in terms of negative emotions, there are important individual differences in the reactivity of positive emotions as well (Spinrad et al., 2004).
Emotional reactivity of patients with BD and MDD deviates from population norms at the behavioral as well as the neural level (Phillips & Swartz, 2014). In everyday life, patients with BD report stronger emotions than healthy individuals (Gruber et al., 2013). These amplified emotions are also reflected in altered neural activity: Early studies in patients with BD report hyperactivity of the amygdala toward emotional stimuli in general (for a review see Townsend & Altshuler, 2012). The amygdala plays a key role in emotion processing, specifically in the detection and perception of stimulus salience and potential threats. Importantly and selectively for positive emotions, patients with BD show elevated amygdala as well as striatal and medial prefrontal cortical activity, alongside decreased effective connectivity from the orbitofrontal cortex to the amygdala (de Almeida et al., 2009; Phillips & Swartz, 2014). These results indicate specific alterations of amygdala activity in BD patients responding to positive stimuli. Abnormal activity in these emotion-processing circuitries is also evident during emotionally neutral, cognitive tasks, suggesting that BD patients are more sensitized to detecting emotional salience (Phillips & Swartz, 2014). This might make it harder for them to distinguish personally relevant from irrelevant situations and thus impede efficient interaction with their social and physical environment. This view is affirmed by a study investigating the interference of emotional material with cognitive tasks (Kanske et al., 2013): When confronted with emotional distractions during a mental arithmetic task, BD patients show more slowing in their reaction times than healthy individuals. Here, a look at the neural level reveals that this behavioral slowing is also accompanied by task-related hyperactivation of parietal attention-related brain regions, suggesting an increased emotional distractibility that also exacerbates neuropsychological impairments in BD.
Like patients with BD, patients with MDD show heightened limbic activity toward emotional stimuli (Delvecchio et al., 2012; Kanske & Kotz, 2012). More specifically, patients with MDD exhibit mood-congruent abnormalities in emotion-processing circuitries comprising the amygdala, insula, putamen as well as the cingulate gyrus and orbitofrontal cortex. Toward negative stimuli, MDD patients show hyperactivation of these regions and a hypoactivation toward positive stimuli (Stuhrmann et al., 2011). Another feature specific to emotional reactivity in MDD is a relative hypoactivation of sensorimotor cortices (Delvecchio et al., 2012), indicating reduced sensory processing of emotional stimuli.
In sum, both patient groups show heightened limbic activity, which might indicate sensitization toward emotional salience and elevated emotional reactivity. While both patients show elevated limbic activity when exposed to negative emotional material, patients with BD also exhibit increased activity in limbic as well as striatal brain areas toward positive emotional stimuli. This implies that BD patients might be generally sensitized to emotional material, whereas reactivity might be specifically increased for negative stimuli in MDD.
Emotion Regulation
To regulate their emotions, individuals use strategies to intensify or attenuate an emotional response (Gross, 1998). These strategies are many and diverse. For example, you might distract yourself after a bad date to reduce feelings of rejection by looking at animal photos, or you might choose to ruminate instead, both of which affect your negative emotional state (Kanske et al., 2011; Nolen-Hoeksema et al., 2008). Efforts to organize these strategies have led to classifications based on, for example, motivational aspects (Koole, 2009), the degree of the strategies’ explicitness (Braunstein et al., 2017), their (mal–)adaptivity (Aldao et al., 2014), or at which point in a perception-evaluation-action cycle the regulation takes place (Gross, 1998, 2015a, 2015b). Although emotion regulation enables individuals to control their emotions, emotion regulation is not always a volitional process but includes components that occur unconsciously and automatically. Models of emotion regulation that take this into account distinguish between processes that take place, on the one hand, planned, consciously, controlled, and volitionally (i. e., explicit emotion regulation), and processes that occur, on the other hand, spontaneously, unconsciously, and automatically (i. e., implicit emotion regulation; Gyurak et al., 2011).
Compared to healthy individuals, both MDD and BD patients report increased spontaneous use of maladaptive strategies to regulate their emotions (Dodd et al., 2019; Gruber et al., 2012; Joormann & Stanton, 2016; Visted et al., 2018). Rumination, suppression, and avoidance are such maladaptive strategies, because they usually provide insufficient emotion regulation or even exacerbate emotional distress (Aldao & Nolen-Hoeksema, 2012; Dodd et al., 2019; Joormann & Stanton, 2016). For example, a patient might tell you that they lie in bed in the evening and grumble about an argument they had during the day, even though they want to sleep. In this case, their emotion regulation is insufficient, and they suffer sleep deprivation as an additional negative consequence. In addition to the frequent use of maladaptive strategies, spontaneous use of implicit emotion regulation appears to be less effective in both patient groups (Gruber et al., 2012; Joormann & Stanton, 2016); hence, MDD patients use adaptive emotion-regulation strategies less frequently than healthy individuals (Visted et al., 2018). In contrast, findings in BD patients are inconsistent, ranging from decreased to increased spontaneous use of adaptive strategies (Gruber et al., 2012; Gruber et al., 2013; Hay et al., 2015; Rowland et al., 2013). Here, it is particularly important to emphasize that, compared to healthy individuals, BD patients do not show significant, observable impairments at the behavioral level when they are explicitly encouraged to use adaptive emotion-regulation strategies (especially reappraisal) (Dodd et al., 2019; Kurtz et al., 2021), and most studies suggest that this is also true for MDD patients (Diedrich et al., 2016; Ehring et al., 2010; Joormann & Stanton, 2016; Rive et al., 2013). However, when affective disorders patients’ emotion regulation is impaired, it can be altered through training: Through repetition, explicit emotion regulation may become habitual and automatic and thus could have an ameliorative effect on BD and MDD pathology. Neural emotion-regulation models support the hypothesis that repeated use of explicit emotion regulation improves implicit emotion regulation through overlap and coactivation of areas associated with the implicit and explicit control systems (Dixon et al., 2017; Phillips et al., 2008), strengthening the notion that emotion regulation can be enhanced through practice, for example, during psychotherapy.
Next to the behavioral level, patients with affective disorders also show aberrations in their neural emotion-regulation systems. When exposed to emotional stimuli, patients with BD show hypoactivity of the medial and lateral prefrontal regions, suggesting lower control over emotional states (Delvecchio et al., 2012; Phillips & Swartz, 2014). During implicit emotion regulation, patients exhibit lower activity in brain areas associated with implicit emotion regulation, such as the orbitofrontal cortex (Phillips et al., 2008). Interestingly, when explicitly instructed to regulate their emotions, patients with BD show hyperactivity in lateral frontal areas compared to healthy controls, although they do not differ in behavioral performance from healthy participants (Kurtz et al., 2021). This suggests that BD patients might be using increased resources to sufficiently regulate their emotions. Together with the idea of a hyperactive limbic system, this dysregulation of cortical emotion-regulation systems might reflect a reduced inhibitory capacity of increased emotional reactivity in BD patients (Delvecchio et al., 2012; Wolkenstein et al., 2017). Functional connectivity studies have affirmed this view, showing lower inferior frontal cortical activity and less connectivity between the ventrolateral prefrontal cortex (vlPFC) and amygdala during the processing of emotional stimuli across different mood states in BD (Phillips & Swartz, 2014; Townsend et al., 2013).
Compared to those in BD patients, the findings regarding emotion regulation in MDD patients are not as consistent (Rive et al., 2013). However, most studies indicate that patients with MDD show changes in neural activity during emotion regulation which differ from those in patients with BD: During implicit emotion regulation, MDD patients show increased neural activity in areas associated with implicit emotion regulation (Rive et al., 2013). In contrast, during explicit emotion regulation and reappraisal, patients with MDD show lower neural activity in brain regions associated with explicit emotion regulation (Rive et al., 2013), suggesting that emotional reactivity is increased in patients with MDD, but that the patients cannot recruit the neural resources necessary to provide the cognitive control that emotion regulation requires when they are asked to do so.
The role of lower cognitive control in depressive symptoms, which occur in both patient groups, is further corroborated by a study in healthy participants inducing activity changes in the dlPFC as part of the cognitive control network (Wolkenstein et al., 2014). These lead to a depression-like negativity bias in healthy participants, suggesting diminished cognitive control as a possible cause of cognitive biases that are central to the current clinical psychological understanding of affective disorders (Wolkenstein et al., 2014). In addition, both patient groups show neural alterations in regions associated with explicit and implicit cognitive control in concordance with a disrupted connection between these systems (e. g., Johnstone et al., 2007; Kanske et al., 2015; Lois et al., 2017; Zhang et al., 2018; Zheng et al., 2018).
The neural changes associated with emotion regulation in BD and MDD patients provide new insights into emotion processing in affective disorders beyond the observable difficulties at the behavioral level: The commitment of increased resources to enable cognitive control to regulate emotions is matched by increased neural reactivity to emotional stimuli. This dynamic reveals a vicious cycle that further reinforces dysregulated affect: Strong affective responses meet weak cognitive control. Weakening of cognitive control systems, in turn, might likely lead to the negatively biased processing of emotional stimuli, as initial findings in healthy controls suggest.
Social Emotions
Emotions generally occur in social situations. Specifically, social emotions are shaped by social situations, depend on other people’s (expected) behaviors, thoughts, or feelings, and are thus relevant to functioning in all kinds of social interactions, ranging from romantic relationships to being part of a work team or society in general (for a review, see Hareli & Parkinson, 2008). For example, imagine you have eaten the last cookie of a package your colleague bought. Just imagining and hence expecting how your colleague will react to seeing that the last cookie has been eaten can induce guilt. As a social emotion, guilt leads to a cascade of further (secondary) emotions or behaviors in this context: You may try to hide the fact that you ate the last cookie or you might buy the next package of cookies.
Compared to more basic emotions (fear or anger), some social emotions are also referred to as self-conscious emotions, because they involve self-evaluative processes (Tracy et al., 2007), or as moral emotions, since they act as social regulators between one’s own needs and the rights of others (Bastin et al., 2016). In particular, self-blaming moral emotions and their relationship with depression have been studied repeatedly. Examples of self-blaming moral emotions are guilt (responding negatively to recognizing one’s violation of a relevant moral or social standard) and shame (responding negatively to the belief that a transgression of a social standard becomes a definition of the self; see also Bastin et al., 2016; Sheehy et al., 2019).
Self-Blaming Moral Emotions: Shame and Guilt
According to the revised learned helplessness model, overgeneralized blaming of oneself leads to decreased self-esteem, hopelessness, and depression, making a person vulnerable to developing a depressive episode (Abramson et al., 1978). Such overgeneralized self-blame is also associated with elevated levels of self-blaming moral emotions in patients with depression (Green et al., 2013; Zahn et al., 2015a; for a meta-analytic review of depressive symptoms on a subclinical level, see S. Kim et al., 2011; on a clinical level, see Guimón et al., 2007; Highfield et al., 2010). Further, also remitted MDD patients show a tendency to experience more self-blaming moral emotions, whereas they are less likely to experience moral emotions toward others, such as contempt, further supporting the revised learned helplessness model (Green et al., 2013; Zahn et al., 2015a). Several studies have supported the association of shame and guilt with depressive symptoms in healthy individuals (e. g., Hawkins et al., 2019; Young et al., 2016), in addition to some studies corroborating this link in patients with MDD (Belden et al., 2015; Gambin & Sharp, 2018; Green et al., 2012; Pulcu et al., 2014; Zahn et al., 2015b). This suggests that, contrary to previous views that assumed that patients with MDD and individuals vulnerable to developing depression experience a generally high level of negative emotions and a deficit of positive emotions (Watson et al., 1988), there is rather an excess of self-blaming moral emotions (Green et al., 2013). This excess of self-blame renders social emotions and specifically self-blaming moral emotions critical to understanding the development of depressive symptoms.
On a neural level, self-blaming moral emotions, in particular shame and guilt, in patients with MDD have been linked to alterations in temporo-limbic structures (Pulcu et al., 2014). In shame, relative to guilt, participants with remitted MDD exhibited stronger neural activation in the right amygdala and posterior insula compared to healthy controls, even though, on a behavioral level, the unpleasantness of guilt and shame and response times did not differ across groups (Pulcu et al., 2014). In addition, patients with remitted and recurrent MDD show alterations in functional connectivity (Green et al., 2012; Lythe et al., 2015) between the temporal lobe and the subgenual cingulate cortex specifically during the processing of self-blame. Evidence for both reduced and increased connectivity (Lythe et al., 2015) of the right superior anterior temporal lobe with the subgenual cingulate cortex and adjacent septal regions has been reported for self-blaming (e. g., guilt) vs. other-blaming emotions. Here, higher coupling of these regions in one study was linked to a higher risk for recurrence of depression, linking the neural processing of self-blame to a more difficult course of disease (Lythe et al., 2015). This idea is further corroborated by recent evidence that this connectivity can be altered with neurofeedback methods in patients with remitted MDD (Zahn et al., 2019), reducing recurrence risk.
Additionally, guilt and shame have been linked to expected social rejection; these findings are in line with results on the hyperactivity of the insula in individuals with MDD in the context of social rejection (for a review, see Kupferberg et al., 2016).
Taken together, the findings indicate that amplified self-blaming moral emotions might be a feature of depression and, in particular, relapse into depression. This idea is also supported by the finding that functional and structural changes in the insula are associated with increased self-blame, on the one hand, but also with relapses into depression, on the other hand (Lemke et al., 2021; Zaremba et al., 2018). This similarity suggests that self-blame may be a way of processing emotions that is associated with vulnerability toward and recurrence of depression. In this context, the experience of social rejection may provide a transdiagnostic risk factor that may cause the overgeneralized bias to blame oneself for failure. In the long run, this bias then may induce excessive self-blame, ultimately leading to the development of depressive symptoms – but most importantly, reduced treatment response and, hence, relapse once depression is manifest.
Compared to patients with MDD, BD patients show a lower propensity for shame and guilt: Concerning manic symptom severity, BD patients show higher levels of trait shame and lower propensity for guilt than healthy controls (Highfield et al., 2010). Comparable findings on shame have been reported in another study with a small subsample of bipolar patients (Guimón et al., 2007), showing higher shame proneness in MDD than in BD patients but no differences in levels of guilt.
Taking into consideration that there is only a limited number of studies investigating self-blaming moral emotions in patients with BD, the summarized evidence indicates that guilt and shame possibly alleviate with remission but amplify severity during depressive episodes in BD. This is highly relevant, since it has been suggested that patients experience more shame after a (hypo–)manic episode (e. g., when realizing that one’s behavior has been transgressive; Fletcher et al., 2013). High self-blame after a (hypo–)manic episode then possibly promotes the development of a depressive episode, once again pointing toward a relevant relationship between self-blaming emotions and the future development of depressive symptoms.
Empathy
Next to the division into self- and other-blaming moral emotions: Social emotions can also be classified as complementary or isomorphic to the emotion of the social interaction partner (Kanske, 2018). Examples of complementary social emotions would be compassion (also referred to as empathic concern; the experience of warm, caring feelings for another person: see Kim et al., 2020), empathic distress, but also guilt and shame (Bastin et al., 2016; Sheehy et al., 2019). In contrast, feeling empathy goes along with sharing the affective state of another, thereby forming an isomorphic representation of the other’s affective state within oneself (Kanske, 2018). You might share the feelings of sadness when a patient reports to you that they are devastated, because no one wants to go out with them, even though you have not been rejected yourself. After this initial sharing of the sadness of your patient, empathy may also transform into a complementary emotion such as compassion. In this case, you might experience care and warmth for the patient further motivating prosocial behavior, such as focusing your next intervention on this experience of rejection. During empathy, the valence of the shared emotion resembles the observed emotion (e. g., sadness). While compassion increases positive affect, empathic distress – another complementary social emotion – is an aversive emotional response that can follow affect sharing, which, contrary to compassion, promotes self-oriented withdrawal behavior. In this case, you would tend to your affective state and lose focus on the particular intervention during therapy.
Higher levels of empathic distress have been observed in patients with MDD (see Kupferberg et al., 2016; Schreiter et al., 2013). More recent studies support these findings (Banzhaf et al., 2018), showing heightened empathic distress in MDD patients, and a weak, positive relationship between empathy and depressive symptoms in healthy individuals (Bennik et al., 2019). During pain observation (vs. seeing nonpainful videos), patients with MDD show lower pain ratings than healthy individuals (Fujino et al., 2014), suggesting diminished affect recognition or lower empathy in patients with MDD.
On a neural level, patients with MDD show greater activation in the left IFG (vlPFC) and less activation in the left middle cingulate cortex and right somatosensory-related cortices than healthy participants during pain observation (Fujino et al., 2014). Comparing neural activity during rest with task-specific activity, mothers with high compared to low depressive symptomatology show lower neural activity in the orbital and medial frontal cortex while empathizing with different children’s facial expressions (Lenzi et al., 2016). When empathizing with emotional facial expressions or with their own child, mothers reporting more depressive symptoms exhibit lower neural activity in the precuneus, higher decrease of neural activity in the orbito and medial frontal cortex, and also increased right amygdala reactivity during both task activity and rest, indicating an emotional dysregulation independent of empathic processes.
Regarding patients with BD, there is only limited evidence on empathic processing, and most studies use self-report measures of empathy. In general, higher empathic distress levels for BD patients were identified than in healthy individuals (Cusi et al., 2010; Derntl et al., 2012; Seidel et al., 2012; Shamay-Tsoory et al., 2009). During acute manic episodes, BD patients show additionally higher empathy than patients with BD in a depressive episode and controls (Bodnar & Rybakowski, 2017), which may also play a role in their increased emotional distractibility (Kanske et al., 2013).
Overall, the findings suggest a higher self-focus in patients with affective disorders in the context of empathy. These patients may concentrate on coping with their own (empathic) distress rather than recognizing and processing another’s emotional response. At the neural level, higher activity in regions associated with emotional reactivity (amygdala) and emotion regulation (IFG, vlPFC) might reflect higher empathic distress, the regulation of which requires increased resources. Lower activity in the precuneus and orbito-medial PFC might also indicate a lower utility of perspective-taking in social situations when confronted with the affective reactions of others, but also a reduced self-other distinction. Self-other distinction denotes the capacity to differentiate between one’s representations of sensations, perceptions, emotions, and actions and those of others (Lamm et al., 2016). Thereby, self-other distinction enables perspective-taking and the experience of compassion (de Guzman et al., 2016; de Waal, 2008). Lower activity in the MCC and somatosensory cortex indicates reduced sharing of affect, suggesting that patients with MDD, although they experience higher empathic distress, may experience reduced sharing of the affective response of the other, perhaps to protect themselves from further distress. However, the reduced sharing of affect may not only shield oneself from empathic suffering, but also from the contagion of positive feelings from others that would alleviate one’s own depressed mood.
Compassion
Since patients with MDD experience increased empathic distress, most studies hypothesize lower compassion in patients with MDD than in healthy people (also referred to as empathic concern). However, results predominantly indicate no differences for compassion between MDD patients and controls (for a review, see Schreiter et al., 2013; but see also Cusi et al., 2011; Ekinci & Ekinci, 2016). The rare findings of lower compassion could also be explained by a higher self-focus in MDD patients (see Kupferberg et al., 2016; Schreiter et al., 2013). Moreover, a negative correlation of compassion with illness duration, but not depression severity, has been reported (Ekinci & Ekinci, 2016), indicating that lower compassion may be a particular feature of chronic depression. In MDD patients, compassion may be linked with self-blaming moral emotions such as guilt and then turn into empathic distress, a negative affective state. Thus, individuals with MDD might not differ regarding their compassionate response to others at first, but, through arising feelings of guilt, transform the initially adaptive response to sharing negative affect into empathic distress rather than compassion.
In contrast to self-blame, self-compassion appears to help patients who must cope with MDD: High dispositional compassion predicts lower depressive symptomatology in total and lower levels of depression-related complaints (negative attitude, performance difficulties, somatic complaints) in early adulthood (Saarinen et al., 2019). A negative age-interaction effect indicates that this association might be stronger in early adulthood and weaken over time. Thus, not taking dispositional compassion in MDD patients into account may also explain inconsistent findings regarding the experience of compassion in patients.
Interestingly, despite these inconsistencies, fear of compassion, that is, fear of being treated compassionately, of showing compassion to others, of experiencing compassion toward others and / or toward oneself, seems to be associated with depression: Compared to healthy individuals, those with depression reported greater fear of receiving compassion from others and fear of being compassionate toward oneself (Merritt & Purdon, 2020). High fear of compassion in interaction with high self-criticism has recently also been associated with higher depression levels in a healthy sample (Hart et al., 2020).
In patients with BD, again, there is only limited evidence in this regard. Two studies revealed that patients with BD report lower compassion compared to patients with MDD and control participants (Derntl et al., 2012; Gruber et al., 2009). However, other studies did not find that BD patients and control subjects differ in compassion (Cusi et al., 2010; Seidel et al., 2012; Shamay-Tsoory et al., 2009). Here, heterogeneity in defining and measuring different social emotions, particularly empathy, empathic distress, and compassion, as they are interwoven processes, may further limit a conclusive analysis.
In summary, there are initial indications that depressive symptoms are connected with higher fear of compassion and lower levels of compassion in MDD patients. A comparison of MDD with BD patients revealed that BD patients experience less compassion than MDD patients.
Research hitherto predominantly indicates that patients with affective disorders experience negative self-blaming social emotions such as guilt and shame more frequently than healthy controls, while less frequently exhibiting self- and other-related positive social emotions, such as compassion. In patients with MDD, changes in the fronto-temporo-limbic regions seem to be associated with changes in social affect, in particular with self-blaming moral emotions. Compared to the extensive research investigating the neural underpinnings of basic emotion processing, there is a lack of research investigating neural correlates of social emotions in affective disorders (Figure 1 depicts an overview of the neural alterations related to emotional reactivity, emotion regulation, and social emotion processing in affective disorders). This is highly important, considering that social emotions are crucial to human interaction and affective disorders yield difficulties in daily social interaction, resulting in hampered social functioning (Hirschfeld et al., 2000). Further, high levels of self-blaming social emotions and lower levels of compassion have been associated with a heightened vulnerability to developing depressive symptoms as well as a higher recurrence of depression. Targeting the processing of social emotions might enhance the understanding of their contribution to affective symptomatology and may render a possibility to alleviate negative treatment outcomes.
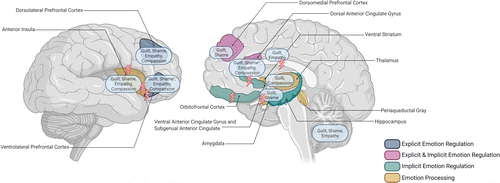
Implications for the Psychotherapy of Affective Disorders
Based on the notion that automatic thoughts and appraisals of situations trigger and maintain pathological affect, cognitive therapy for affective disorders aims to alter these cognitive processes to reduce pathological affect. Here, affect is first monitored over time, and the relationships between automatic thoughts, appraisal, and emotional response are decoded across situations (Beck & Rush, 1979).
Cognitive therapy identifies automatic negative thoughts that give rise to negative affect in response to everyday events. For example, the situation “missing the bus” might trigger the automatic thought “I am not in control of my life“, which causes feelings of “helplessness”. In the next step, automatic thoughts are disputed and replaced with realistic and more nuanced thoughts to reduce negative affect. Thus, cognitive therapy predominantly modifies cognitive processes, based on the notion that this modification ultimately alters affective states and therefore only indirectly aims to alter emotion processing. Although this cognitive approach is helpful for many patients, some patients do not respond to cognitive therapy. These patients become very adept at refuting their negative thoughts and beliefs and practice their new positive thoughts in daily life but report that they still feel their negative thoughts and beliefs are true. If a patient reports that, despite sufficient practice and against their better judgment, they still feel that their irrational automatic thoughts are true, focusing on the emotional processes may be particularly valuable to their psychotherapy.
In this final section of our narrative review article, we now report on two more recent treatment approaches that build on the changes in emotional reactivity, emotion regulation, and social affect and that are also apparent in patients with affective disorders: emotion-regulation therapy and compassion-focused therapy.
Emotion-regulation therapy (ERT) is an effective treatment for patients with affective disorders who exhibit intense emotional reactivity and high self-centeredness, more specifically a high self-referentiality, which may manifest in an increased tendency to worry or ruminate (Mennin & Fresco, 2013, 2014; Northoff, 2007). ERT is based on the notion that increased emotional reactivity is associated with increased difficulty in reducing psychological distress, resulting in negative feelings accumulating rather than dissipating. In this review article, we previously reported that patients with affective disorders exhibit increased emotional reactivity accompanied by reduced (cognitive) control, which could ultimately result in a reduced capacity to efficiently regulate emotions. We also summarized that increased self-referentiality may represent an adaptation to protect the patient from being inundated with negative emotion from the environment, for example, from others, but it may also lead to a further build-up of negative affect within the self, exacerbating the difficulty in reducing psychological distress (Mennin & Fresco, 2013). In this context, worry and rumination can be a self-referential way to regulate emotional stress.
On a neural level, increased self-referentiality has been represented by functional alterations of the anterior insula and amygdala as regions that are hyperactivated in patients with affective disorders reflecting their elevated emotional reactivity, and the medial prefrontal cortex (mPFC) as part of the cognitive control network (Renna et al., 2017). Another important feature of self-referentiality is a decreased connectivity between cognitive control areas and regions associated with elevated emotional reactivity, which may also indicate a decreased inhibitory capacity of prefrontal control areas toward limbic and striatal emotion-processing regions – a feature observed in patients with bipolar disorder as well (Lythe et al., 2015; Phillips & Swartz, 2014). To date, few studies have examined whether ERT has the potential to both reduce increased self-referentiality and emotional distress, and to alter the functional brain changes associated with this symptomatology. These studies mainly examined patients suffering from many worries as well as depressive symptoms, such as patients with depression and / or generalized anxiety disorder. Based on the idea that increased self-referentiality is a maintenance mechanism of strong emotional reactivity, this target group is certainly of particular interest for initial studies. However, because patients with bipolar disorder also show strong emotional reactivity and alterations of the neural systems underlying emotional reactivity, an investigation of the efficacy of ERT in this study population might be useful. Indeed, in previous studies, ERT was shown to reduce emotional distress, worrying, and depressive symptoms, on the one hand, and to attenuate functional disturbances of the mPFC, anterior insula, and amygdala as well as the connectivity between these regions in patients with MDD and / or generalized anxiety disorder, on the other hand (Fresco et al., 2017; Scult et al., 2019). Because patients with BD also exhibit similar neural changes during emotion processing, this approach may also prove appropriate for BD patients (see also Figure 2).
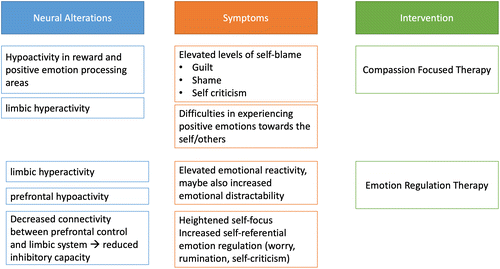
Taken together, the tenor of the studies we reviewed supports the idea that the use of ERT may be a beneficial treatment option for patients with affective disorders when standard procedures, such as cognitive therapy, fail to result in symptom improvement. For practitioners, the increased self-referentiality represents an observable feature that may help identify patients who could benefit from ERT. ERT targets specifically motivational and emotion-regulatory processes, such as self-referential processing (worry, rumination, self-criticism) and behavioral processes (avoidance, reassurance-seeking, compulsive behavior) (Mennin & Fresco, 2014). During two therapeutic phases, a mindful and flexible reaction toward emotions, on the one hand, and a proactive and value-oriented behavioral approach on the other hand, are conveyed to the patient. In a nutshell, these interventions aim at reducing the automatic generation of negative affective states and increasing cognitive control. Both intervention phases combined lead to high and long-term reduction of distress (Renna et al., 2017).
Compassion-focused therapy (CFT) uses the inherently positive social emotion of compassion to promote self- and other-related positive emotions (Förster & Kanske, 2021; Gilbert, 2009, 2014). In this review article, we already reported that patients with affective disorders may experience elevated levels of self-blaming emotions rather than other related negative emotions. Further, we elaborated that relapse into depression, increased self-blame, and the experience of social rejection have all been associated with functional and structural alterations of the insula. These common neural changes of depressive symptomatology, experienced social rejection, and heightened self-related negative emotions also fit very well into the disorder model of CFT: This therapy is based on the idea that psychopathology arises from unbalanced affect regulation systems. Three systems should be in balance: the drive system (benefit: finding resources), the threat defence system (protection), and the soothing system. Because of early negative social experiences (such as emotional neglect, abuse, but also bullying), the soothing system does not experience sufficient positive stimulation, which leads to social situations activating the threat defence system more quickly (Gilbert, 2009). As a result, patients with early negative interpersonal experiences are more vulnerable to social rejection and more poorly able to experience reassuring positive emotions, such as warmth and contentment, toward the self and others. Specifically, in this review, we summarized how patients with an affective disorder exhibit an imbalance between negative and positive emotions toward themselves (Gilbert, 2009).
In cognitive therapy, we observe that patients with this strong tendency toward self-criticism and self-blame are cognitively able to generate positive thoughts about themselves, but that they still feel their negative self-beliefs are true and thus may show a lower treatment response (Rector et al., 2000). Thus, according to this basic tenet of CFT, patients with affective disorders who report early aversive experiences and who find it difficult to show positive emotions toward themselves and others respond particularly well to CFT. On a neural level, practicing compassion in healthy individuals activates the neural circuits of the soothing system (ventral striatum, medial orbitofrontal cortex, subgenual cingulate cortex; see Kim et al., 2011; Kim et al., 2020). This activation helps to upregulate positive affect and thereby to countervail and regulate negative affect (Engen & Singer, 2015; Klimecki et al., 2013, 2014). Structural neuroimaging insights furthermore reveal increases in cortical thickness of fronto-insular regions after the extended practice of compassion in healthy individuals (Valk et al., 2017), indicating an increased capacity to regulate emotions.
As described above, patients with affective disorders show alterations of striatal and subgenual anterior cingulate activity also associated with lower treatment response (Mayberg et al., 1997; Redlich et al., 2016) and aberrant emotion regulation (Rive et al., 2013, see Figure 2). However, in contrast to ERT research, no empirical studies to date have shown that CFT also reduces these brain changes in individuals with psychopathology (Förster & Kanske, 2021).
On a behavioral level, CFT is highly effective and has been evaluated positively for increasing positive affect and alleviating distress, rumination, shame, and self-criticism (Bluth & Neff, 2018; Ehret et al., 2015; MacBeth & Gumley, 2012; Neff, 2003; Neff & Dahm, 2015). Notably, this is observable specifically in patients with affective disorders (Frostadottir & Dorjee, 2019; Gilbert, 2009). Since high levels of self-criticism, shame, and rumination imply maladaptive emotion regulation (Ehret et al., 2015), these results can also be interpreted as an improved emotion regulation via the practice of compassion.
In summary, preliminary evidence suggests that fostering compassion during CFT and targeting emotion regulation during ERT can specifically reduce distress in patients with affective disorders that respond only insufficiently to treatment as usual. Informed by the summarized neural models, both approaches display a high potential to alleviate increased emotional reactivity, aberrant emotion regulation, and increased negative social affect. We focused on neural changes related to altered emotional reactivity, emotion regulation, and social emotions in affective disorders to derive therapeutic strategies for these disorders. However, because changes in, for example, emotion regulation and underlying neural systems have transdiagnostic relevance and are also observable in relatives of patients with various mental disorders (see, e. g., Taylor & Liberzon, 2007; Thorsen et al., 2019), the potentials of the mechanisms for the prevention and treatment of other mental disorders should also be examined.
Conclusions
Following and building on a detailed description of the neural changes associated with emotional reactivity, emotion regulation, and social affect, this review described two treatment approaches for affective disorders informed by neural models. Emotion-regulation therapy aims at reducing emotional reactivity by targeting self-referentiality (as observable in increased rumination or worry) and promoting the connectivity between emotion processing (amygdala, insula) and cognitive control networks. In contrast, compassion-focused therapy aims at upregulating activity in neural systems associated with positive emotions (ventral striatum, medial orbitofrontal cortex, and subgenual anterior cingulate cortex) in patients who experienced emotional neglect and abuse sensitizing them toward social rejection, and who fail to generate positive social emotions leaving them with an unbearable amount of self-blame and self-criticism.
Our review suggests that incorporating elements of these two treatment approaches presents new perspectives for the treatment of affective disorders by specifically targeting the alterations of neural systems underlying emotional experiences in affective disorders.
Literatur
(1978). Learned helplessness in humans: Critique and reformulation. Journal of Abnormal Psychology, 87 (1), 49 – 74. https://doi.org/10.1037/0021-843x.87.1.49
(2014). Adaptive and maladaptive emotion regulation strategies: Interactive effects during CBT for social anxiety disorder. Journal of Anxiety Disorders, 28 (4), 382 – 389. https://doi.org/10.1016/j.janxdis.2014.03.005
(2012). When are adaptive strategies most predictive of psychopathology? Journal of Abnormal Psychology, 121 (1), 276 – 281. https://doi.org/10.1037/a0023598
(2018). Interacting and dissociable effects of alexithymia and depression on empathy. Psychiatry Research, 270, 631 – 638. https://doi.org/10.1016/j.psychres.2018.10.045
(2016). Feelings of shame, embarrassment and guilt and their neural correlates: A systematic review. Neuroscience & Biobehavioral Reviews, 71, 455 – 471. https://doi.org/10.1016/j.neubiorev.2016.09.019
(1979). Cognitive therapy of depression (13th ed.). Guilford.
(2015). Anterior insula volume and guilt: Neurobehavioral markers of recurrence after early childhood major depressive disorder. JAMA Psychiatry, 72 (1), 40 – 48. https://doi.org/10.1001/jamapsychiatry.2014.1604
(2019). The relation between empathy and depressive symptoms in a Dutch population sample. Journal of Affective Disorders, 242, 48 – 51. https://doi.org/10.1016/j.jad.2018.08.008
(2018). New frontiers in understanding the benefits of self-compassion. Self and Identity, 17 (6), 605 – 608. https://doi.org/10.1080/15298868.2018.1508494
(2017). Increased affective empathy in bipolar patients during a manic episode. Revista Brasileira de Psiquiatria, 39 (4), 342 – 345. https://doi.org/10.1590/1516-4446-2016-2101
(2017). Explicit and implicit emotion regulation: A multi-level framework. Social Cognitive and Affective Neuroscience, 12 (10), 1545 – 1557. https://doi.org/10.1093/scan/nsx096
(2010). Altered self-report of empathic responding in patients with bipolar disorder. Psychiatry Research, 178 (2), 354 – 358. https://doi.org/10.1016/j.psychres.2009.07.009
(2011). Altered empathic responding in major depressive disorder: Relation to symptom severity, illness burden, and psychosocial outcome. Psychiatry Research, 188 (2), 231 – 236. https://doi.org/10.1016/j.psychres.2011.04.013
(2009). Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biological Psychiatry, 66 (5), 451 – 459. https://doi.org/10.1016/j.biopsych.2009.03.024
(2016). Self-other control processes in social cognition: From imitation to empathy. Philosophical Transactions of the Royal Society B: Biological Sciences, 371 (1686), 20150079 https://doi.org/10.1098/rstb.2015.0079
(2008). Putting the altruism back into altruism: The evolution of empathy. Annual Review of Psychology, 59, 279 – 300. https://doi.org/10.1146/annurev.psych.59.103006.093625
(2012). Common and distinct neural correlates of emotional processing in Bipolar Disorder and Major Depressive Disorder: A voxel-based meta-analysis of functional magnetic resonance imaging studies. European Neuropsychopharmacology, 22 (2), 100 – 113. https://doi.org/10.1016/J.EURONEURO.2011.07.003
(2012). How specific are emotional deficits? A comparison of empathic abilities in schizophrenia, bipolar and depressed patients. Schizophrenia Research, 142(1 – 3), 58 – 64. https://doi.org/10.1016/j.schres.2012.09.020
(2016). Self-compassion enhances the efficacy of explicit cognitive reappraisal as an emotion regulation strategy in individuals with major depressive disorder. Behaviour Research and Therapy, 82, 1 – 10. https://doi.org/10.1016/j.brat.2016.04.003
(2017). Emotion and the prefrontal cortex: An integrative review. Psychological Bulletin, 143 (10), 1033 – 1081. https://doi.org/10.1037/bul0000096
(2019). Emotion regulation strategies in bipolar disorder: A systematic and critical review. Journal of Affective Disorders, 246, 262 – 284. https://doi.org/10.1016/j.jad.2018.12.026
(2015). Examining risk and resilience factors for depression: The role of self-criticism and self-compassion. Cognition & Emotion, 29 (8), 1496 – 1504. https://doi.org/10.1080/02699931.2014.992394
(2010). Emotion regulation and vulnerability to depression: Spontaneous versus instructed use of emotion suppression and reappraisal. Emotion, 10 (4), 563 – 572. https://doi.org/10.1037/a0019010
(2016). Relationship between empathic responding and its clinical characteristics in patients with major depressive disorder. Düşünen Adam: The Journal of Psychiatry and Neurological Sciences, 2 (29), 145 – 154. https://doi.org/10.5350/DAJPN2016290206
(2015). Compassion-based emotion regulation up-regulates experienced positive affect and associated neural networks. Social Cognitive and Affective Neuroscience, 10 (9), 1291 – 1301. https://doi.org/10.1093/scan/nsv008
(2013). High-risk behaviour in hypomanic states. Journal of Affective Disorders, 150 (1), 50 – 56. https://doi.org/10.1016/j.jad.2013.02.018
(2020). Brain structural correlates of alexithymia in patients with major depressive disorder. Journal of Psychiatry & Neuroscience, 45 (2), 117 – 124. https://doi.org/10.1503/jpn.190044
(2021). Exploiting the plasticity of compassion to improve psychotherapy. Current Opinion in Behavioral Sciences, 39, 64 – 71. https://doi.org/10.1016/j.cobeha.2021.01.010
(2017). Distinct functional connectivities predict clinical response with emotion-regulation therapy. Frontiers in Human Neuroscience, 11, 86. https://doi.org/10.3389/fnhum.2017.00086
(2019). Effects of mindfulness based cognitive therapy (MBCT) and compassion-focused therapy (CFT) on symptom change, mindfulness, self-compassion, and rumination in clients with depression, anxiety, and stress. Frontiers in Psychology, 10, 1099. https://doi.org/10.3389/fpsyg.2019.01099
(2014). Altered brain response to others׳ pain in major depressive disorder. Journal of Affective Disorders, 165, 170 – 175. https://doi.org/10.1016/j.jad.2014.04.058
(2018). The relations between empathy, guilt, shame and depression in inpatient adolescents. Journal of Affective Disorders, 241, 381 – 387. https://doi.org/10.1016/j.jad.2018.08.068
(2009). Introducing compassion-focused therapy. Advances in Psychiatric Treatment, 15 (3), 199 – 208. https://doi.org/10.1192/apt.bp.107.005264
(2014). The origins and nature of compassion-focused therapy. The British Journal of Clinical Psychology, 53 (1), 6 – 41. https://doi.org/10.1111/bjc.12043
(1995). Relapse and impairment in bipolar disorder. The American Journal of Psychiatry, 152 (11), 1635 – 1640. https://doi.org/10.1176/ajp.152.11.1635
(2012). Guilt-selective functional disconnection of anterior temporal and subgenual cortices in major depressive disorder. Archives of General Psychiatry, 69 (10), 1014 – 1021. https://doi.org/10.1001/archgenpsychiatry.2012.135
(2013). Proneness to decreased negative emotions in major depressive disorder when blaming others rather than oneself. Psychopathology, 46 (1), 34 – 44. https://doi.org/10.1159/000338632
(1998). The emerging field of emotion regulation: An integrative review. Review of General Psychology, 2 (3), 271 – 299. https://doi.org/10.1037/1089-2680.2.3.271
(2015a). Emotion regulation: Current status and future prospects. Psychological Inquiry, 26 (1), 1 – 26. https://doi.org/10.1080/1047840X.2014.940781
(2015b). The extended process model of emotion regulation: Elaborations, applications, and future directions. Psychological Inquiry, 26 (1), 130 – 137. https://doi.org/10.1080/1047840X.2015.989751
(2009). Do positive emotions predict symptomatic change in bipolar disorder? Bipolar Disorders, 11 (3), 330 – 336. https://doi.org/10.1111/j.1399-5618.2009.00679.x
(2012). When trying is not enough: Emotion regulation and the effort-success gap in bipolar disorder. Emotion, 12 (5), 997 – 1003. https://doi.org/10.1037/a0026822
(2013). Real-world emotion? An experience-sampling approach to emotion experience and regulation in bipolar I disorder. Journal of Abnormal Psychology, 122 (4), 971 – 983. https://doi.org/10.1037/a0034425
(2007). Shame, sensitivity to punishment and psychiatric disorders. The European Journal of Psychiatry, 21 (2), 124 – 133. https://doi.org/10.4321/s0213-61632007000200004
(2011). Explicit and implicit emotion regulation: A dual-process framework. Cognition & Emotion, 25 (3), 400 – 412. https://doi.org/10.1080/02699931.2010.544160
(2008). What’s social about social emotions? Journal for the Theory of Social Behaviour, 38 (2), 131 – 156. https://doi.org/10.1111/j.1468-5914.2008.00363.x
(2020). Insecure striving, self-criticism, and depression: The prospective moderating role of fear of compassion from others. Mindfulness, 11 (7), 1699 – 1709. https://doi.org/10.1007/s12671-020-01385-8
(2019). Parental adjustment following pediatric burn injury: The role of guilt, shame, and self-compassion. Journal of Pediatric Psychology, 44 (2), 229 – 237. https://doi.org/10.1093/jpepsy/jsy079
(2015). Choosing how to feel: Emotion regulation choice in bipolar disorder. Emotion, 15 (2), 139 – 145. https://doi.org/10.1037/emo0000024
(2017). Decreased medial prefrontal cortex activation during self-referential processing in bipolar mania. Journal of Affective Disorders, 219, 157 – 163. https://doi.org/10.1016/j.jad.2017.04.065
(2010). An investigation into the experience of self-conscious emotions in individuals with bipolar disorder, unipolar depression and non-psychiatric controls. Clinical Psychology & Psychotherapy, 17 (5), 395 – 405. https://doi.org/10.1002/cpp.674
(2000). Social functioning in depression: A review. The Journal of Clinical Psychiatry, 61 (4), 268 – 275. https://doi.org/10.4088/jcp.v61n0405
(2007). Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. The Journal of Neuroscience, 27 (33), 8877 – 8884. https://doi.org/10.1523/JNEUROSCI.2063-07.2007
(2016). Examining emotion regulation in depression: A review and future directions. Behaviour Research and Therapy, 86, 35 – 49. https://doi.org/10.1016/j.brat.2016.07.007
(2018). The social mind: Disentangling affective and cognitive routes to understanding others. Interdisciplinary Science Reviews, 43 (2), 115 – 124. https://doi.org/10.1080/03080188.2018.1453243
(2011). How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral Cortex, 21 (6), 1379 – 1388. https://doi.org/10.1093/cercor/bhq216
(2013). Neural correlates of emotional distractibility in bipolar disorder patients, unaffected relatives, and individuals with hypomanic personality. The American Journal of Psychiatry, 170 (12), 1487 – 1496. https://doi.org/10.1176/appi.ajp.2013.12081044
(2012). Effortful control, depression, and anxiety correlate with the influence of emotion on executive attentional control. Biological Psychology, 91 (1), 88 – 95. https://doi.org/10.1016/j.biopsycho.2012.04.007
(2015). Impaired regulation of emotion: Neural correlates of reappraisal and distraction in bipolar disorder and unaffected relatives. Translational Psychiatry, 5,
e497 . https://doi.org/10.1038/tp.2014.137(2020). The neurophysiological basis of compassion: An fMRI meta-analysis of compassion and its related neural processes. Neuroscience & Biobehavioral Reviews, 108, 112 – 123. https://doi.org/10.1016/j.neubiorev.2019.10.023
(2011). Shame, guilt, and depressive symptoms: A meta-analytic review. Psychological Bulletin, 137 (1), 68 – 96. https://doi.org/10.1037/a0021466
(2013). Functional neural plasticity and associated changes in positive affect after compassion training. Cerebral Cortex, 23 (7), 1552 – 1561. https://doi.org/10.1093/cercor/bhs142
(2014). Differential pattern of functional brain plasticity after compassion and empathy training. Social Cognitive and Affective Neuroscience, 9 (6), 873 – 879. https://doi.org/10.1093/scan/nst060
(2009). The psychology of emotion regulation: An integrative review. Cognition & Emotion, 23 (1), 4 – 41. https://doi.org/10.1080/02699930802619031
(2016). Social functioning in major depressive disorder. Neuroscience & Biobehavioral Reviews, 69, 313 – 332. https://doi.org/10.1016/j.neubiorev.2016.07.002
(2021). Deficits in explicit emotion regulation in bipolar disorder: A systematic review. International Journal of Bipolar Disorders, 9 (1), 15 https://doi.org/10.1186/s40345-021-00221-9
(2016). From shared to distinct self-other representations in empathy: Evidence from neurotypical function and socio-cognitive disorders. Philosophical Transactions of the Royal Society B: Biological Sciences, 371 (1686), 20150083 https://doi.org/10.1098/rstb.2015.0083
(2021). The course of disease in major depressive disorder is associated with altered activity of the limbic system during negative emotion processing. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. Online prepublication https://doi.org/10.1016/j.bpsc.2021.05.008
(2016). Mothers with depressive symptoms display differential brain activations when empathizing with infant faces. Psychiatry Research: Neuroimaging, 249, 1 – 11. https://doi.org/10.1016/j.pscychresns.2016.01.019
(2017). Large-scale network functional interactions during distraction and reappraisal in remitted bipolar and unipolar patients. Bipolar Disorders, 19 (6), 487 – 495. https://doi.org/10.1111/bdi.12512
(2001). An evaluation of the absolute and relative stability of alexithymia in patients with major depression. Psychotherapy and Psychosomatics, 70 (5), 254 – 260. https://doi.org/10.1159/000056263
(2015). Self-blame-selective hyperconnectivity between anterior temporal and subgenual cortices and prediction of recurrent depressive episodes. JAMA Psychiatry, 72 (11), 1119 – 1126. https://doi.org/10.1001/jamapsychiatry.2015.1813
(2012). Exploring compassion: A meta-analysis of the association between self-compassion and psychopathology. Clinical Psychology Review, 32 (6), 545 – 552. https://doi.org/10.1016/j.cpr.2012.06.003
(1997). Cingulate function in depression: A potential predictor of treatment response. Neuroreport, 8 (4), 1057 – 1061. https://doi.org/10.1097/00001756-199703030-00048
(2013). What, Me Worry and Ruminate About DSM-5 and RDoC? The importance of targeting negative self-referential processing. Clinical Psychology, 20 (3), 258 – 267. https://doi.org/10.1111/cpsp.12038
(2014). Emotion-regulation therapy. In Handbook of emotion regulation (2nd ed., pp. 469 – 490). Guilford.
(2020). Scared of compassion: Fear of compassion in anxiety, mood, and non‐clinical groups. British Journal of Clinical Psychology, 59 (3), 354 – 368. https://doi.org/10.1111/bjc.12250
(2012). Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990 – 2010: A systematic analysis for the Global Burden of Disease Study 2010. The Lancet, 380 (9859), 2197 – 2223. https://doi.org/10.1016/S0140-6736(12)61689-4
(2003). Self-compassion: An alternative conceptualization of a healthy attitude toward oneself. Self and Identity, 2 (2), 85 – 101. https://doi.org/10.1080/15298860309032
(2015).
Self-compassion: What it is, what it does, and how it relates to mindfulness . In B. D. OstafinM. D. RobinsonB. P. Meier (Eds.), Handbook of mindfulness and self-regulation (pp. 121 – 137). Springer. https://doi.org/10.1007/978-1-4939-2263-5_10(2008). Rethinking rumination. Perspectives on Psychological Science, 3 (5), 400 – 424. https://doi.org/10.1111/j.1745-6924.2008.00088.x
(2007). Psychopathology and pathophysiology of the self in depression: Neuropsychiatric hypothesis. Journal of Affective Disorders, 104(1 – 3), 1 – 14. https://doi.org/10.1016/j.jad.2007.02.012
(2019). Alexithymia predicts poorer social and everyday functioning in schizophrenia and bipolar disorder. Psychiatry Research, 273, 218 – 226. https://doi.org/10.1016/j.psychres.2019.01.033
(2003). Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological Psychiatry, 54 (5), 515 – 528. https://doi.org/10.1016/S0006-3223(03)00171-9
(2008). A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13 (9), 829, 833 – 57. https://doi.org/10.1038/mp.2008.65
(2014). A critical appraisal of neuroimaging studies of bipolar disorder: Toward a new conceptualization of underlying neural circuitry and a road map for future research. The American Journal of Psychiatry, 171 (8), 829 – 843. https://doi.org/10.1176/appi.ajp.2014.13081008
(2014). Increased amygdala response to shame in remitted major depressive disorder. PLoS ONE, 9 (1),
e86900 . https://doi.org/10.1371/journal.pone.0086900(2000). Self-criticism and dependency in depressed patients treated with cognitive therapy or pharmacotherapy. Cognitive Therapy and Research, 24 (5), 571 – 584. https://doi.org/10.1023/A:1005566112869
(2016). Prediction of individual response to electroconvulsive therapy via machine learning on structural magnetic resonance imaging data. JAMA Psychiatry, 73 (6), 557 – 564. https://doi.org/10.1001/jamapsychiatry.2016.0316
(2017). Emotion-regulation therapy: A mechanism-targeted treatment for disorders of distress. Frontiers in Psychology, 8, 98. https://doi.org/10.3389/fpsyg.2017.00098
(2013). Neural correlates of dysfunctional emotion regulation in major depressive disorder: A systematic review of neuroimaging studies. Neuroscience & Biobehavioral Reviews, 37 (10), 2529 – 2553. https://doi.org/10.1016/j.neubiorev.2013.07.018
(2013). Cognitive regulation of negative affect in schizophrenia and bipolar disorder. Psychiatry Research, 208 (1), 21 – 28. https://doi.org/10.1016/j.psychres.2013.02.021
(2019). The relationship of dispositional compassion for others with depressive symptoms over a 15-year prospective follow-up. Journal of Affective Disorders, 250 (250), 354 – 362. https://doi.org/10.1016/j.jad.2019.03.029
(2013). Empathy in adults with clinical or subclinical depressive symptoms. Journal of Affective Disorders, 150 (1), 1 – 16. https://doi.org/10.1016/j.jad.2013.03.009
(2019). Changes in functional connectivity following treatment with emotion-regulation therapy. Frontiers in Behavioral Neuroscience, 13, 10. https://doi.org/10.3389/fnbeh.2019.00010
(2012). Risk or resilience? Empathic abilities in patients with bipolar disorders and their first-degree relatives. Journal of Psychiatric Research, 46 (3), 382 – 388. https://doi.org/10.1016/j.jpsychires.2011.11.006
(2009). Neuropsychological evidence of impaired cognitive empathy in euthymic bipolar disorder. The Journal of Neuropsychiatry and Clinical Neurosciences, 21 (1), 59 – 67.
(2019). An examination of the relationship between shame, guilt and self-harm: A systematic review and meta-analysis. Clinical Psychology Review, 73, 101779. https://doi.org/10.1016/j.cpr.2019.101779
(1973). The prevalence of “alexithymic“ characteristics in psychosomatic patients. Psychotherapy and Psychosomatics, 22 (2), 255 – 262. https://doi.org/10.1159/000286529
(2015). Prevention of relapse and recurrence in adults with major depressive disorder: Systematic review and meta-analyses of controlled trials. The International Journal of Neuropsychopharmacology, 19 (2), pyv076 https://doi.org/10.1093/ijnp/pyv076
(2004). The relation of children’s everyday nonsocial peer play behavior to their emotionality, regulation, and social functioning. Developmental Psychology, 40 (1), 67 – 80. https://doi.org/10.1037/0012-1649.40.1.67
(2011). Facial emotion processing in major depression: A systematic review of neuroimaging findings. Biology of Mood & Anxiety Disorders, 1 (1), 10 https://doi.org/10.1186/2045-5380-1-10
(2007). Neural correlates of emotion regulation in psychopathology. Trends in Cognitive Sciences, 11 (10), 413 – 418. https://doi.org/10.1016/j.tics.2007.08.006
(2019). Emotion regulation in obsessive-compulsive disorder, unaffected siblings, and unrelated healthy control participants. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4 (4), 352 – 360. https://doi.org/10.1016/j.bpsc.2018.03.007
(2012). Emotion processing and regulation in bipolar disorder: A review. Bipolar Disorders, 14 (4), 326 – 339. https://doi.org/10.1111/j.1399-5618.2012.01021.x
(2013). Frontal-amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biological Psychiatry, 73 (2), 127 – 135. https://doi.org/10.1016/j.biopsych.2012.06.030
(Eds.). (2007). The self-conscious emotions: Theory and research. Guilford. Retrieved from http://site.ebrary.com/lib/alltitles/docDetail.action?docID=10267367
(2017). Structural plasticity of the social brain: Differential change after socio-affective and cognitive mental training. Science Advances, 3 (10),
e1700489 . https://doi.org/10.1126/sciadv.1700489(2018). Emotion regulation in current and remitted depression: A systematic review and meta-analysis. Frontiers in Psychology, 9, 756. https://doi.org/10.3389/fpsyg.2018.00756
(1988). Positive and negative affectivity and their relation to anxiety and depressive disorders. Journal of Abnormal Psychology, 97 (3), 346 – 353. https://doi.org/10.1037/0021-843x.97.3.346
(2017). Impaired cognitive control over emotional material in euthymic bipolar disorder. Journal of Affective Disorders, 214, 108 – 114. https://doi.org/10.1016/j.jad.2017.03.007
(2014). Induction of a depression-like negativity bias by cathodal transcranial direct current stimulation. Cortex, 59, 103 – 112. https://doi.org/10.1016/j.cortex.2014.07.011
(2017). Decreased empathy response to other people’s pain in bipolar disorder: Evidence from an event-related potential study. Scientific Reports, 7 (1), 1 – 11. https://doi.org/10.1038/srep39903
(2016). Shame and guilt-proneness as mediators of associations between general causality orientations and depressive symptoms. Journal of Social and Clinical Psychology, 35 (5), 357 – 370. https://doi.org/10.1521/jscp.2016.35.5.357
(2015a). Negative emotions toward others are diminished in remitted major depression. European Psychiatry, 30 (4), 448 – 453. https://doi.org/10.1016/j.eurpsy.2015.02.005
(2015b). The role of self-blame and worthlessness in the psychopathology of major depressive disorder. Journal of Affective Disorders, 186, 337 – 341. https://doi.org/10.1016/j.jad.2015.08.001
(2019). Blame-rebalance fMRI neurofeedback in major depressive disorder: A randomised proof-of-concept trial. NeuroImage. Clinical, 24, 101992. https://doi.org/10.1016/j.nicl.2019.101992
(2018). Association of brain cortical changes with relapse in patients with major depressive disorder. JAMA Psychiatry, 75 (5), 484 – 492. https://doi.org/10.1001/jamapsychiatry.2018.0123
(2018). Altered frontal-amygdala effective connectivity during effortful emotion regulation in bipolar disorder. Bipolar Disorders, 20 (4), 349 – 358. https://doi.org/10.1111/bdi.12611
(2018). Incapacity to control emotion in major depression may arise from disrupted white matter integrity and OFC-amygdala inhibition. CNS Neuroscience & Therapeutics, 24 (11), 1053 – 1062. https://doi.org/10.1111/cns.12800



