Age Differences in Learning-Related Neurophysiological Changes
Measures of Brain Activity, Eye Tracking, Skin Conductance, Heart Rate, and Respiration
Abstract
Abstract: Research in young adults has demonstrated that neurophysiological measures are able to provide insight into learning processes. However, to date, it remains unclear whether neurophysiological changes during learning in older adults are comparable to those in younger adults. The current study addressed this issue by exploring age differences in changes over time in a range of neurophysiological outcome measures collected during visuomotor sequence learning. Specifically, measures of electroencephalography (EEG), skin conductance, heart rate, heart rate variability, respiration rate, and eye-related measures, in addition to behavioral performance measures, were collected in younger (Mage = 27.24 years) and older adults (Mage = 58.06 years) during learning. Behavioral responses became more accurate over time in both age groups during visuomotor sequence learning. Yet, older adults needed more time in each trial to enhance the precision of their movement. Changes in EEG during learning demonstrated a stronger increase in theta power in older compared to younger adults and a decrease in gamma power in older adults while increasing slightly in younger adults. No such differences between the two age groups were found on other neurophysiological outcome measures, suggesting changes in brain activity during learning to be more sensitive to age differences than changes in peripheral physiology. Additionally, differences in which neurophysiological outcomes were associated with behavioral performance on the learning task were found between younger and older adults. This indicates that the neurophysiological underpinnings of learning may differ between younger and older adults. Therefore, the current findings highlight the importance of taking age into account when aiming to gain insight into behavioral performance through neurophysiology during learning.
Learning processes have most commonly been examined using behavioral measures (Luu et al., 2009; Tinga et al., 2020b; Webb et al., 1966). Non-invasive measurements of neurophysiology, however, have been demonstrated to be a valuable alternative measure of learning, providing insight not only into the outcome of learning but also into the learning process itself (Tinga et al., 2019). These non-invasive neurophysiological measures include measures of brain activity such as electroencephalography (EEG) as well as measures of peripheral physiology including heart rate, electrodermal activity, and respiration. Eye tracking measures are another type of non-invasive neurophysiological measure. Neurophysiology has been demonstrated to have high potential as a measure of learning (Krigolson et al., 2015; Leff et al., 2011; Tinga et al., 2019). Measures of neurophysiology respond in a predictable manner to cognitive demand or mental effort (Antonenko et al., 2010; Borghini et al., 2014; Charles & Nixon, 2019; Hogervorst et al., 2014; Tinga et al., 2020b). As mental effort increases, parasympathetic inhibition decreases, and sympathetic activation increases (Berntson et al., 1991), reflected in measures of peripheral physiology and eye-related measures. These changes are paralleled by changes in the central nervous system such as in oscillations in the EEG signal with alpha and theta oscillations generally respectively increasing and decreasing with decreasing demands (Antonenko et al., 2010; Brouwer et al., 2012). Because learning can be considered as a change in the cognitive demands a task induces when the task becomes less difficult and processing of the task becomes less effortful (e.g. Fairclough et al., 2005; Schneider & Chein, 2003; Tinga et al., 2020b), neurophysiology has the potential to provide insight into learning.
A recent meta-analysis of 113 experiments demonstrated that learning-related changes in neurophysiology and behavior are differentially affected by individual differences and task-related aspects (Tinga et al., 2019). Participant age showed to be an important individual difference that influenced how neurophysiology changed over time during learning. Specifically, changes in neurophysiology decreased as participants’ age increased. Even though these effects of age were found for neurophysiology, no such effects were found for changes in behavioral outcomes during learning. Most studies examining neurophysiological changes during learning have included the common relatively young adult participant population, with the mean age of participants in the 113 included experiments in the meta-analysis of Tinga et al. (2019) being 28.58 years. How these findings translate to an older population however remains unclear. Indeed, it has been questioned to what extent learning-related changes in neurophysiology occur in older adults, although this issue has received scant attention in the literature (Alain & Snyder, 2008).
Furthermore, most of the experiments, at least those included in the meta-analysis by Tinga et al. (2019), focus (solely) on changes in brain activity during learning. We have emphasized the importance of considering a multitude of neurophysiological measures (Tinga et al., 2019, 2020a, 2020b). As most studies focused on brain activity outcome measures, it is unclear whether the effect of age reported in our meta-analysis (Tinga et al., 2019) translates to other neurophysiological outcome measures.
It is important to gain insight into how neurophysiological outcome measures, both brain activity outcome measures and peripheral ones, change during learning in older compared to younger populations when one wants to assess learning through these measures in people of varying ages in practice. In educational settings, the outcome of learning is normally only assessed at the end of learning. Neurophysiology provides the opportunity to measure learning over time during learning itself. It can support predicting the outcome of learning and deeper insights into learning can be obtained due to the possibility to record multi-channel data at a high sample rate (Tinga et al., 2020b). When age differences in learning-related neurophysiological changes are well understood they can be taken into account to ensure valid assessment.
However, only a few experimental studies compared learning-related changes in neurophysiology between younger and older adults. Most of the existing studies focused on changes in event-related potentials (ERPs) obtained through EEG measurements. Alain and Snyder (2008) examined changes in ERP amplitudes during learning to distinguish different auditory vowels in both younger (Mage = 24.0 years) and older adults (Mage = 67.8 years). While both age groups showed comparable learning gains incorrect responses and reaction times, changes in ERPs during learning did differ between the age groups. Some changes in ERPs were present in both younger and older adults, while other effects were only present in younger or older adults. For one effect of learning on ERPs that was present in both age groups in the temporal lobe, the authors reported the learning-related changes to be larger in younger adults. Likewise, Eppinger and colleagues (2008) and Eppinger and Kray (2011) examined changes in ERPs during learning from feedback with differing validity or with either monetary gains or losses respectively in younger (Mage = 20.8 years; 22.1 years) and older adults (Mage = 68.5 years; 69.7 years). Performance accuracy increased over time during learning from feedback with high validity (≥ 80%) in a comparable way in younger and older adults. Learning rates with both monetary gains and losses were higher in younger than in older adults. Regarding age differences in changes in neurophysiology during learning, only younger adults demonstrated a change in ERPs at frontocentral sites in both studies. In two experiments (Pietschmann et al., 2008, 2011), younger adults (Mage = 22.0 years; 25.8 years) and older adults (Mage = 65.9 years; 65.8 years) learned stimulus-response associations. Between the two age groups, only one significant difference in behavioral learning was demonstrated in only one of the two experiments (Pietschmann et al., 2011). This demonstrated that older adults needed more trials than younger adults to learn the stimulus-response associations. Regarding age differences in changes in neurophysiology during learning, younger adults demonstrated changes in ERP amplitudes over time at frontocentral sites, while older participants did not (Pietschmann et al., 2011) or did only for one ERP amplitude measure (Pietschmann et al., 2008).
All in all, these studies demonstrate that changes in ERPs during learning are generally larger in younger compared to older adults. Such findings are important in providing insight into age differences in learning-related neurophysiological changes. Yet, specific response measures such as ERPs can only be computed by presenting many similar stimuli that are able to elicit an ERP that can be reliably time-locked to those stimuli. These stimuli can be presented on a primary task, or via a repeatedly presented probe irrelevant to the task (Gevins & Smith, 2003). These specific response measures are therefore difficult to apply in practice, outside the lab, and may be challenging to integrate into learning technologies.
A small number of studies examined non-invasive neurophysiological outcomes reflecting more general cognitive changes during learning that can be measured continuously without time-locking neurophysiology to many repeating stimuli. One such study by Neider and colleagues (2010) examined changes in eye movements over time during visual search learning in younger (Mage = 20 years) and older adults (Mage = 65 years). However, they did not find any difference between these two age groups. A study examining general changes in power bands in the EEG signal by Lopez-Loeza and colleagues (2016) tested younger (Mage = 21.50) and older adults (Mage = 52.75 years) on a visuospatial learning task. The older adults showed a pattern of generally lower theta power and higher gamma power in frontal and temporal brain areas. However, changes over time, which would be reflective of learning processes specifically, were not examined in this study.
Although previous work points to age differences in learning-related changes in neurophysiology, there is only a limited number of studies on this topic, and studies that do exist mostly focus on very specific outcome measures (such as ERPs in EEG). Therefore, it remains unclear whether learning improvements in behavioral performance in older adults are paralleled by changes in a range of neurophysiological outcome measures being reflective of more general cognitive changes and whether this is comparable to changes observed in younger adults.
The current study aimed to explore how a range of neurophysiological outcome measures change during learning in older compared to younger adults. Behavioral measures and multiple non-invasive neurophysiological measures, including EEG, skin conductance level, heart rate, heart rate variability, respiration rate, and eye tracking metrics were collected. These measures were recorded in both younger and older adults during implicit visuomotor sequence learning. The acquisition of motor sequences is essential throughout our lives and for healthy aging, as we learn new motor skills as well as when we need to modify previously learned skills (Fitzroy et al., 2021; King et al., 2013; Moisello et al., 2009). The current study selected the Serial Reaction Time Task (SRT; Nissen & Bullemer, 1987). This is a standard paradigm for assessing visuomotor sequence learning and it is used often in research studying differences between learning in younger and older adults (Fitzroy et al., 2021). In this task, participants respond to stimuli successively appearing at different locations by making a spatially corresponding response. Unbeknownst to the participants, the required responses follow a continuous complex sequence.
Generally, both younger and older adults show marked improvements in behavioral performance over time during visuomotor sequence learning, both due to implicitly learning the sequence of responses and due to learning the principles of the task. On average, however, younger adults learn better than older adults due to age-related decreases in cognitive functioning (King et al., 2013). Therefore, in the current study, we expected behavioral performance to increase over time during task learning, with the learning performance of younger adults exceeding that of older adults. Yet, as it is currently unclear how neurophysiology changes during learning in older compared to younger adults, learning-related changes in neurophysiology in both age groups were examined in an exploratory fashion. This exploration aims to support the next steps toward the assessment of learning through neurophysiology in practice.
Methods
Participants
Seventeen young adults (age range = 22–30 years, Mage = 27.24 ± 2.17 years; 13 females) and 17 older adults (age range = 55–62 years, Mage = 58.06 ± 2.08 years; 11 females) participated in the current study. All participants were employees at Tilburg University and were included if they reported no current cardiovascular disease, neurological disorder, and lung disease (following Tinga et al., 2020a, 2021). None of the participants reported colorblindness.
The number of participants was estimated based on previous studies, as well as on a power analysis. The number of participants in the experimental studies of Alain and Snyder (2008), Eppinger et al. (2008), Eppinger and Kray (2011), Lopez-Loeza et al. (2016), and Pietschmann et al. (2008, 2011) discussed above ranged between 12 and 18 participants per group. Using the lowest effect size (partial eta squared, ηp2) for reported effects regarding interactions between time and age on neurophysiological outcome measures in these experimental studies (namely a ηp2 of .06) and a priori power analyses for repeated measures, within-between interaction F tests in G*Power 3 (Faul et al., 2007), with an error probability of 0.05 and a power of 0.95, indicated that a total sample size of 10 for 2 different groups would ensure sufficient power.
Apparatus and Measures
The task was presented on a desktop monitor (BenQ Zowie XL2540, 1,920 × 1,080 pixels, 240 Hz refresh rate) running Unity 3D (version 2017.4.1). Participants used a joystick (Ultimarc UltraStik 360, mounted on a table 16 cm in front of the participants’ body midline) to interact with the task. The coordinates of the cursor’s position controlled by the joystick were recorded at 90 Hz.
Three-lead electrocardiography (ECG), respiration, and electrodermal activity (EDA) were measured continuously throughout the experiment at 2,000 Hz using BioNomadix wireless systems (BN-RSPEC and BN-PPGED, BIOPAC Systems, Inc.). The ECG signal was bandlimited online from 0.05 Hz to 150 Hz and the respiration and EDA signals were both bandlimited online from DC to 10 Hz. These signals were collected using AcqKnowledge 5.0 (BIOPAC Systems, Inc.) software running on a computer exclusively dedicated to collecting the ECG, respiration, and EDA data.
Eye tracking data were collected continuously throughout the experiment at 60 Hz using a desktop eye tracker (REDn scientific, SMI) and the iView REDn Scientific 4.4.26 (SMI) and Experiment Center 3.7.68 (SMI) software packages.
Nine-channel (Fz, F3, F4, Cz, C3, C4, Pz, P3, and P4) EEG signals were measured continuously throughout the experiment at 256 Hz using a wireless B-Alert X10 system (Advanced Brain Monitoring). The EEG signals were collected using AcqKnowledge 5.0 software running on a computer exclusively dedicated to collecting the EEG data.
Learning Task and Stimuli
Following Tinga and colleagues (2020a, 2021), the learning task (see Figure 1) was a version of the SRT in which responses to targets need to be made using arm movements. The stimuli to which participants responded were eight white target circles presented on a dark gray background. The target circles had a diameter of 108 pixels and were evenly spaced apart at 360 pixels from the center of the screen. Additionally, a white circle with a diameter of 108 pixels was presented in the center of the screen. Participants moved the cursor (black small circle with a diameter of 33 pixels) with the joystick from the center of the screen to one of the targets and back to the center. A target was selected by one of the target circles turning light gray. Target selection was always in synchrony with a 160 ms tone (presented via headphones) at an interval of 1 s. If participants hit the selected target correctly with the cursor, the target turned green. When a participant made an incorrect choice by either hitting a non-selected target or hitting the selected target too late, the target that was hit turned red.
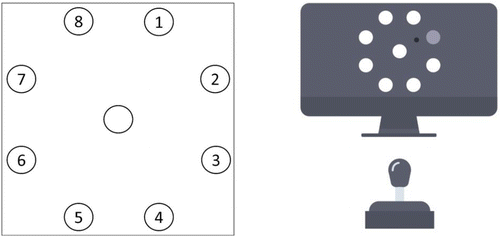
The task consisted of 8 learning blocks, each with 128 trials of 1 s. Targets for movements were selected in a repeating sequence of 16 elements in which each target was selected twice. Two such sequences were used, with each participant being presented with one of the two sequences, counterbalanced between participants (Figure 1, left). No random sequence was included for the purpose of the current study, following Tinga and colleagues (2021). Moreover, previous research has demonstrated that neurophysiology is reflective of more general learning processes and not specifically of sequence learning (Tinga et al., 2020a).
Procedure
After obtaining written and verbal informed consent, participants filled out a questionnaire on demographics (i.e., age and gender). The EEG, ECG, respiration, and EDA sensors were placed on the participant, the eye tracker was calibrated, and data collection of the neurophysiological measures was started. First, a baseline of the neurophysiology of three minutes was collected in which participants were presented with a black fixation cross on a dark gray background. Participants were instructed to sit calmly and still while keeping their eyes open while fixating on the fixation cross.
The learning task (Figure 1, right) was started after 16 practice trials. Participants were instructed to move the cursor using the joystick as fast and as accurately as possible and to reverse sharply within the target circle back to the middle. A second and third baseline of the neurophysiology of three minutes was collected after the fourth and eighth blocks of the learning task. A schematic overview of the current study’s procedure is depicted in Figure 2.

Following Moisello and colleagues (2009) and Tinga and colleagues (2020a, 2021), after completing the learning task participants were asked to indicate the (most common) order in which the circles were selected as a target for each of the two parts of the task separately to test their declarative knowledge of the presented sequences. To this aim, participants were shown the schematic depiction in Figure 1 on the left and were asked to write this order down by referring to the numbers in the figure. Participants were instructed to guess if they did not recall the order. Experimental sessions, including setting up and removing the sensors, took approximately 70 min per participant.
Data Processing and Analyses
Processing of Behavioral Outcome Measures
Multiple behavioral outcome measures were computed in order to gain better insight into what aspects of the learning process showed differences between the two age groups. We followed Moisello and colleagues (2009) in computing a range of behavioral outcomes per trial: (1) Whether the response on a trial was correct, that is, when a movement was initiated from the middle and when the selected target was hit within 1 s; (2) time from the start of the trial until the start of movement (onset time, OT); (3) time from the start of movement until the end of the movement (movement time, MT); (4) sum of the absolute OT and MT (response time, RT); (5) maximum speed of displacement between OT and end of the movement (peak velocity, PV); (6) linear distance from the endpoint of the movement and the center of the target (spatial error, SE); (7) the area in which the cursor moved divided by the squared movement length (normalized movement area, NMA).
Processing of Neurophysiological Outcome Measures
Electrocardiography
A finite impulse response (FIR) high-pass filter at 1 Hz and an FIR low-pass filter at 35 Hz were applied to the recorded ECG signal. QRS complexes were automatically identified in the ECG signal using AcqKnowledge 5.0. The identified complexes were manually checked and adjusted when needed. Based on the marked QRS complexes heart rate and the root mean square of successive differences (RMSSD) as a measure of heart rate variability wase computed.
Respiration
An FIR low-pass filter at 1 Hz was applied to the recorded respiration signal. Breaths per minute were determined using AcqKnowledge 5.0.
Electrodermal Activity
The recorded EDA signal was resampled to 50 Hz. Median smoothing with 1 s windows and an FIR low-pass filter at 1 Hz were applied on the resampled signal. The skin conductance level was computed as the average EDA.
Eye Tracking
The program BeGaze 3.7.59 (SMI) was used to process the collected eye-tracking signal. For each sample, the pupil diameter in millimeters and the category of the sample (i.e., blink, fixation, or saccade) were determined.
Electroencephalography
The FieldTrip EEG processing toolbox (version 2019-10-08; Oostenveld et al., 2011) was used in the software program MATLAB (version R2019a) to process the collected EEG signal. Data were low-passed filtered at 60 Hz after which automatic EOG and muscle artifact rejection was performed. Cut-offs for EOG and muscle artifact rejection were set at z = 4 and z = 8 and performed inside frequency bands of 1–15 Hz and 100–125 Hz, respectively. The relative delta (1–3 Hz), theta (4–7 Hz), alpha (8–13 Hz), beta (15–25 Hz), and gamma frequency (30–60 Hz) for each channel were computed with the multitaper method using Hanning tapers. The range of frequency bands was based on those used in earlier research (Arnal & Kleinschmidt, 2017; Tesche & Karhu, 2000; Wrobel, 2000).
Statistical Analyses
A declarative knowledge score was determined for each participant by computing the maximum overlap (in percentages) between the order that was indicated at the end of the experiment and the real sequence (of 16 targets) that was presented. A maximum permitted declarative knowledge score of 40% (i.e., having a maximum overlap between the real and indicated order of 7 or more) was set following earlier work (Curran & Keele, 1993; Moisello et al., 2009; Tinga et al., 2020a, 2021; Willingham et al., 1989). A maximum score was set to ensure that the included data was all from participants that learned the SRT implicitly.
Behavioral and neurophysiological outcomes were averaged for each block. Averages for OT, MT, RT, PV, SE, and NMA were determined for correct trials only. The average heart rate, heart rate variability, breaths per minute, skin conductance level, pupil diameter, number and duration of blinks, number and duration of saccades, number and durations of fixations, and relative power in the EEG delta, theta, alpha, beta, and gamma frequency bands for the whole scalp, frontal, central, and parietal electrodes were computed for the baselines and each block.
In order to examine age differences in learning-related neurophysiological changes, the current study’s analyses included two factors: age (younger vs.older adults) and block (the task’s eight learning blocks). Both the main effects and the interaction between the factors were tested. These tests allowed for (1) examining general age differences that are reflected in overall differences during the execution of the task both for behavioral and neurophysiological outcome measures and (2) examining learning-related age differences that are reflected in changes over time during the execution of the task both for behavioral and neurophysiological outcome measures. Following the recommendation of Figner and colleagues (2022), the interpretation of the pattern of statistically significant interactions was based on exploring means and figures showing the pattern, rather than running additional statistical tests. Finally, in order to explore whether neurophysiology can be utilized to provide insight into behavioral performance during learning in both age groups, the relationship between neurophysiological outcomes and behavioral outcomes over all blocks was tested for younger and older adults.
All statistical analyses were performed with linear mixed-effects models analyses using lme in R (R Core Team, 2017) with the subject as a random factor.
The analyses included a relatively high number of comparisons as the current study included a range of neurophysiological outcomes. One may hold the opinion that this means that p-values must be adjusted for multiple comparisons. Yet, as 31 neurophysiological outcome measures are included, correcting for multiple comparisons means that p-values for these outcome measures need to be smaller than .0016 (.0500/31) for the effect to be considered statistically significant. As the effects of learning on neurophysiology had an average p-value of .0472 in the included studies in the meta-analysis of Tinga and colleagues (2019), adjusting the p-values in the current study is expected to lead to a high type II error rate and is therefore undesirable. This is in line with the recommendation of Rothman (1990) to not adjust for multiple comparisons in order to make fewer type II errors and therefore to prevent not detecting possibly important findings. Similarly, Althouse (2016) pointed out that adjusting for multiple comparisons reduces the likelihood of associations being found in larger studies compared to small studies. Perneger (1998) even deemed such adjustments “deleterious to sound statistical inference.” Althouse (2016) recommended reporting methods and results in detail and letting readers use their own judgment about the relative weight of the findings. In line with these recommendations, and in order to avoid a Type II error, we, therefore, applied no adjustment for multiple comparisons. However, we raise this issue to make the reader aware of this decision when interpreting significant results.
Results
With declarative knowledge scores all below 40%, none of the participants needed to be excluded from further analyses based on their declarative knowledge score. The average declarative score was 20.94% (SD = 7.64%, range = 12.50–37.50%) and 16.56% (SD = 5.39%, range = 12.50–31.25%) for the younger and older adults, respectively. For artifact removal in the EEG data, the maximum percentage of samples that were discarded in a block/baseline was 15.21%, with an average of 2.64% of samples discarded after artifact removal. ECG data of one participant were excluded due to one of the sensors detaching during the experiment and the EDA data of one participant was excluded due to sensor placement not being possible on the fingers of the non-dominant hand. Eye tracking data of two participants were not collected due to technical issues. All remaining eye-tracking data were of high quality, with all baselines and blocks including at least 75% non-missing samples, with an average of 98.59% of non-missing samples.
In sum, data of all 34 participants (17 young and 17 older adults) were included for analyses on behavioral, EEG, and respiration data. After the exclusion of participants as mentioned in the preceding paragraph, data of 33 participants (16 young and 17 older adults) were included for analyses on ECG data, data of 33 participants (16 young and 17 older adults) were included for analyses on EDA data, and data of 32 participants (15 young and 17 older adults) were included for analyses on eye tracking data.
Behavioral Performance
A general difference was found in overall behavioral performance between younger and older adults. Younger adults had on average 27.77% more correct responses than older adults. Additionally, in younger adults MT was 24.84 ms longer, SE was 194.22 pixels smaller, and NMA was 2.08% larger than in older adults. These results demonstrate that younger adults had more correct and precise responses than older adults while making slightly longer movements covering a larger area. These results are in line with previous research generally demonstrating slower motor responses and slower response times and less accurate responses in older adults (Verhaeghen, 2016; Woods et al., 2015).
Regarding performance changes over blocks in all participants, correct responses and MT indeed increased by 29.04% and 18.92 ms respectively. PV and SE decreased by 282.16 pixels per second and 360.92 pixels, respectively. There was also an effect on NMA, which decreased by 1.27%. Age interacted with block, demonstrating that the increase in correct responses over blocks was larger in older adults than younger adults (an increase of 33.69% in older adults compared to 24.40% in younger adults), with older adults starting and ending with a lower number of correct responses than younger adults. Additionally, while OT and RT increased over blocks in older adults, they decreased in younger adults. SE decreased also more strongly for the older adults, who started with a higher SE than younger adults in the first block and ended with a higher SE than younger adults in the last block. Details of effects (F, p, ηp2) on behavioral outcome measures are presented in Table 1.
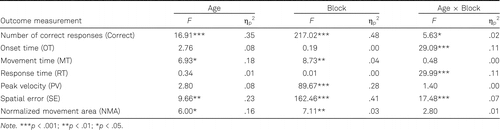
These findings demonstrate that behavioral responses became more correct over blocks, by making movements slower (but not by slowing the total response time) and more precise. Most importantly, these changes over blocks interacted with age, demonstrating that although older adults performed less well than younger adults in general, they did show larger increases in performing correctly and precisely during learning. Yet, older adults became slower over blocks in starting their movements, increasing their total response time, while younger adults became faster. These findings are in line with previous research indicating that older adults prioritize accuracy over speed (Verhaeghen, 2016). While changes in behavioral outcome measures during learning differed between younger and older adults, both demonstrated marked improvements in the number of correct responses over time, indicating sufficient learning in both age groups.
Neurophysiology
General differences between the age groups were found for pupil diameter and central theta power, demonstrating a smaller pupil diameter in older (M = 3.37 mm, SD = 0.41 mm) than in younger adults (M = 3.82 mm, SD = 0.58 mm) and a lower theta power at central sites in older (M = 10.32%, SD = 4.50%) than in younger adults (M = 14.28%, SD = 5.50%). A smaller pupil diameter has been linked to a generally lower effort investment during learning (Takeuchi et al., 2011). These effects could, however, also be linked to differences in physiology due to age, irrespective of cognitive demands during learning, as it has been demonstrated that pupil size decreases with increasing age (Winn et al., 1994). Moreover, general differences between younger (20–30 years) and older (65–75 years) adults in theta power have been reported (Jabès et al., 2021). General age differences in theta power during visuospatial learning have been reported by Lopez-Loeza and colleagues (2016), who also demonstrated lower theta power in older adults compared to younger adults. In order to gain better insight into whether these general differences are specific to the demands of the learning task, we explored age differences in pupil diameter and central theta power during the three baselines as post-hoc tests. The two age groups differed significantly in baseline pupil diameter, F(1, 30) = 6.02, p = .020, ηp2 = .17, but not in central theta power, F(1, 32) = 3.82, p = .059, ηp2 = .11, demonstrating that the general difference in pupil size during the execution of the learning task in the current study indeed is associated with differences between both age groups irrespective of task demands, while not providing such evidence for central theta power.
Many neurophysiological outcome measures changed over blocks during task learning in both age groups. Heart rate and respiration rate decreased by 1.64 beats per minute and 0.36 breaths per minute respectively. Pupil diameter decreased by 0.16 mm, the number of saccades increased by 4.56 saccades per minute and fixation duration increased by 9.09 ms. Total, frontal, central, and parietal EEG theta power increased by 0.99%, 1.06%, 1.00%, and 0.77%, respectively. Total, frontal, central, and parietal EEG alpha power increased by 1.07%, 1.17%, 1.12%, and 0.68%, respectively. Beta power decreased at all sites by 0.43%, at frontal sites with 0.49%, and at central sites by 0.52%. Total, frontal, central, and parietal EEG gamma power decreased by 0.93%, 0.78%, 1.46%, and 0.37%, respectively. To a large extent, these neurophysiological changes seem to be reflective of the task becoming easier over time, associated with a decrease in cognitive effort exertion over time.
In addition, neurophysiological changes during learning differed between the two age groups. Total EEG theta power increased more strongly in older than in younger adults (an increase of 1.56% vs. 0.42%, respectively). This difference was also found for EEG theta power at frontal (an increase of 1.77% vs. 0.35%, respectively), central (an increase of 1.59% vs. 0.41%, respectively), and parietal sites (an increase of 1.08% vs. 0.37%, respectively) specifically. The changes in total EEG theta power are depicted in Figure 3 on the left. The pattern of changes in the total EEG theta power is comparable to the pattern of changes at frontal, central, and parietal sites. From these patterns, it seems that the stronger increase in older adults mainly occurs in the first four blocks. Total gamma power decreased in older adults (by 2.15%), but increased in younger adults (by 0.30%). This effect was also found for EEG gamma power at frontal, central, and parietal sites specifically, demonstrating a decrease in older adults of 2.09%, 2.71%, and 1.38%, respectively. The changes in total EEG gamma power are depicted in Figure 3 on the right. The pattern of changes in the total EEG gamma power is comparable to the pattern of changes at frontal, central, and parietal sites. From these patterns, it appears that the decrease in older participants occurs mainly in the first seven blocks and that the increase in younger participants is rather small. Details of effects (F, p, ηp2) on neurophysiological outcome measures are presented in Table 2.
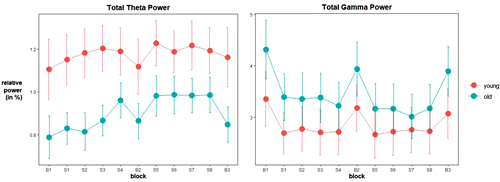
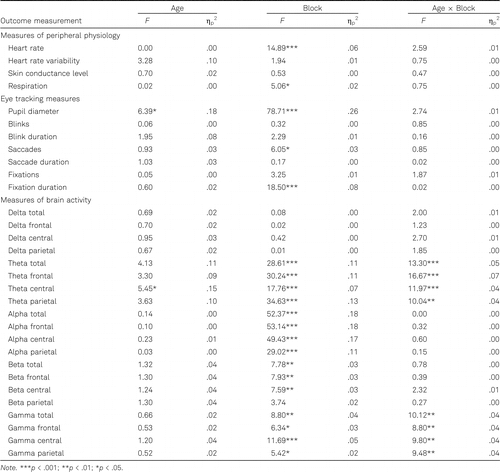
These findings demonstrate that changes in brain activity during learning were different in younger compared to older adults. However, no age differences were found for other neurophysiological outcome measures, suggesting that changes during learning in brain activity are more sensitive to age than changes in peripheral physiology.
Relationship Between Neurophysiology and Behavioral Performance in Younger and Older Adults
Heart rate, pupil diameter, number of blinks, fixation duration, parietal delta power, parietal theta power, and alpha power at all sites were related to at least one behavioral outcome measurement in both younger and older adults. Only in younger adults were heart rate variability, number of saccades, number of fixations, and parietal beta power related to at least one behavioral outcome measurement. Skin conductance level, respiration, delta and theta power at total, frontal and central sites, central beta power, and gamma power at all sites were related to behavioral performance only in older adults. The outcomes (ηp2) of testing the relationship between each behavioral and neurophysiological measurement are reported in Table 3 for the younger adults and in Table 4 for the older adults.
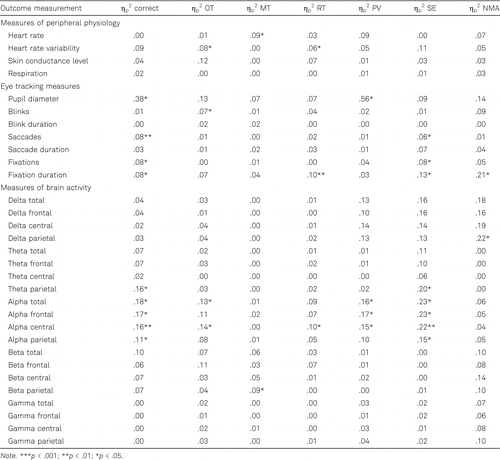
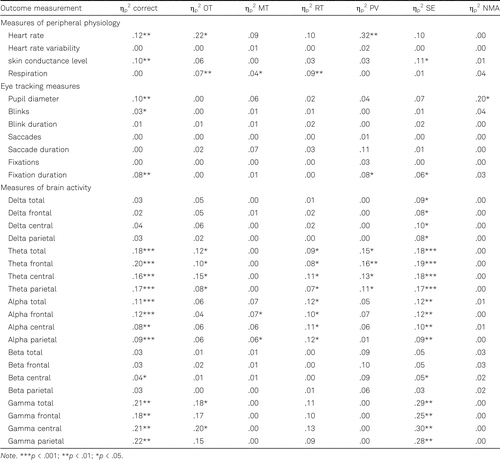
These results suggest that several measures of neurophysiology provide insight into behavioral performance irrespective of age, while other neurophysiological outcomes could provide insight only in younger or older adults. Important to note here, however, is that for all neurophysiological outcome measures that gave insight into behavioral performance for both age groups, none of these outcome measures provided insight into the same behavioral performance metrics in the two groups. Therefore, it seems to be important to take age into account when aiming to gain insight into behavioral performance through neurophysiology during learning.
Discussion
Non-invasive measurements of neurophysiology can provide valuable insights into learning. Studies on this topic, however, have predominantly examined effects in young adults only. Few prior studies have compared learning-related changes in neurophysiology between younger and older adults and those few studies mostly focused on very specific neurophysiological outcome measures. Therefore, it is unclear whether learning in older adults is paralleled by a change in a range of neurophysiological outcome measures being reflective of more general cognitive changes comparable to those observed in younger adults. The current study addressed this issue by presenting both younger (Mage = 27.24 years) and older adults (Mage = 58.06 years) with an implicit visuomotor sequence learning task. During task learning, behavioral measures of learning and multiple non-invasive neurophysiological measures, including EEG, skin conductance level, heart rate, heart rate variability, respiration rate, and eye tracking metrics were collected.
General Age Differences
On the whole, results demonstrated that age differences existed in overall behavioral performance and neurophysiology. Responses were on average more correct and more precise, while movements were slightly longer and covered a larger area, in younger compared to older adults. General age differences also existed in neurophysiology, demonstrating a smaller pupil diameter and lower EEG theta power at central sites in older compared to younger adults. With pupil diameter also being smaller in older adults during the task baselines, the age difference in pupil diameter is probably due to inherent differences in physiology between both age groups and not due to differences in the demands of the learning task. Central power did not differ significantly between the two age groups at baseline, which might reflect that the task induces different levels of mental effort in younger and older adults. This finding is in line with Lopez-Loeza and colleagues (2016), who also found generally lower theta power in older adults compared to younger adults during visuospatial learning, interpreting this as task efficiency and information retrieval skills being higher in younger than older adults.
Age Differences in Learning-Related Changes
Effects related to the process of learning specifically would be revealed by changes over time in the outcomes. Behavioral performance improved over time and these improvements interacted with age. Older adults demonstrated larger enhancements incorrect responses and precision of responses during learning. While responses of younger adults became faster over time, responses of older adults became slower. These findings indicate behavioral learning differed between younger and older adults. Yet, both age groups demonstrated marked improvements in the number of correct responses over time, suggesting sufficient learning in both younger and older adults.
Many neurophysiological outcome measures changed during learning: Heart rate, respiration rate, and pupil diameter decreased, the number of saccades and fixation duration increased, EEG theta and alpha power increased, and EEG beta and gamma power decreased. To a large extent, these neurophysiological changes seem to be reflective of the task becoming easier during learning, reducing cognitive effort over time (Krigolson et al., 2015; Leff et al., 2011; Tinga et al., 2019). Changes in brain activity during learning interacted with age; EEG theta power increased more strongly in older than in younger adults and EEG gamma power decreased in older adults while it increased slightly in younger adults. The findings on theta power suggest that although older participants had a generally lower theta power, the power in this frequency band increases more strongly during learning. As EEG theta and gamma power have both been implicated to play a role in the encoding and retrieval of visuospatial information and visuospatial learning specifically (Lopez-Loeza et al., 2016; Sato & Yamaguchi, 2007), the current findings indicate these processes develop differently over time during learning in younger compared to older adults. Even when behavioral performance is about equal in younger and older adults, different networks of brain regions can be employed by both age groups as demonstrated by studies using functional neuroimaging (Grady & Craik, 2000; Reuter-Lorenz, 2002). This suggests that the brain can be differently employed in older adults in order to perform a task well.
No age differences were demonstrated on other neurophysiological outcome measures, suggesting changes in brain activity during learning to be more sensitive to age differences than changes in peripheral physiology. This finding is important in several respects. First of all, it indicates that the effect of age on neurophysiological changes during learning reported in the meta-analysis by Tinga and colleagues (2019) might mainly be due to the fact that included studies predominantly examined outcome measures of brain activity. This means that age differences might not exist for all neurophysiological changes during learning. Additionally, the current study suggests that not only specific response measures in the EEG, that need to be time-locked to many similar stimuli but also power bands in the EEG, reflecting more general cognitive changes that can be measured continuously during learning, are sensitive to age. These continuous measures of EEG are easier to employ in a wide range of learning settings including settings outside the laboratory.
Relationship Between Neurophysiology and Behavioral Performance
The current study also demonstrated that several neurophysiological measures provided insight into behavioral performance on the learning task in both younger and older adults. These measures included heart rate, pupil diameter, number of blinks, fixation duration, parietal delta power, parietal theta power, and alpha power at all sites. However, several neurophysiological measures provided insight into behavioral performance in only one of the two age groups. These results suggest that several neurophysiological measures provide insight into behavioral performance irrespective of age, while other neurophysiological outcomes could provide insight only in younger or older adults. This indicates that the neurophysiological underpinnings of learning may differ between younger and older adults. Therefore, it seems to be important to take age into account when aiming to gain insight into behavioral performance through neurophysiology during learning.
Limitations and Recommendations for Future Research
In the interpretation of the findings of the current study, it is important to take into account that age differences in learning-related changes in neurophysiology were examined in an exploratory fashion. The findings, therefore, need to be replicated in future studies, preferably also in different age groups. The group of older participants included in the current study was aged 55–62 years with a mean age of about 58 years. Other studies examining age differences as discussed in the introduction included a group of older participants with an average age ranging from 53 years to 70 years with the average age of all studies being about 65 years. As the current study demonstrated age to have an impact on changes in brain activity during learning, an interesting endeavor for future studies would be to additionally include participants of an older age than included in the current study to examine whether findings replicate and/or become more pronounced. At the same time, if studies on neurophysiology in learning compare younger and older adults in light of the workforce, the age groups used in the current study are preferred. Additionally, we recommend future studies to collect more extensive demographic data, such as data on the level of education and type of occupation of the included younger and older participants, to assess age differences while controlling for such demographics. Moreover, studies investigating age differences often include information on participants’ cognitive abilities in addition to information on demographics. For example, Eppinger and Kray (2011) employed two psychometric tests to report fluid and crystallized intelligence in their sample in which they investigated age differences in learning from positive and negative feedback. Such information aids in interpreting reported age differences and is therefore advisable.
Previous work has also demonstrated factors related to the learning task, such as whether the feedback is provided on performance or not and the sensory system in which learning takes place (Fairclough & Roberts, 2011; Tinga et al., 2019), to impact neurophysiological changes during learning. It might therefore be valuable for future work to examine whether an interplay between task-related factors and age exists. Additionally, exploring the implications of the differences in EEG theta and gamma power between younger and older adults during learning in more detail might support the development of interventions during learning in older adults or even in patient groups that deal with cognitive decline. Theta power was related to enhanced behavioral performance in both age groups in the current study. Similarly, previous work (Hanslmayr et al., 2019) has suggested that enhancing theta power through audiovisual stimulation leads to enhanced memory performance. Therefore, intervening on theta power to aid learning in older adults might be especially promising.
Conclusion
Learning is typically assessed based solely on behavioral outcomes, but measures of neurophysiology might be equally valuable. The findings of the current study demonstrated that a non-invasive measure of brain activity during learning shows different changes in younger compared to older adults. Therefore, changes in brain activity during learning might be more sensitive to age differences than changes in peripheral physiology. Additionally, when gaining insight into behavioral performance through neurophysiology during learning, age differences exist. This indicates that the neurophysiological underpinnings of learning may differ between younger and older adults and highlights the importance of taking age into account when assessing learning through neurophysiology.
We would like to thank Anton Sluijtman, Erwin Peters, Maarten Horden, and Peter van Trier for their valuable input in designing and setting up the task.
References
(2008). Age-related differences in auditory evoked responses during rapid perceptual learning. Clinical Neurophysiology, 119(2), 356–366. https://doi.org/10.1016/j.clinph.2007.10.024
(2016). Adjust for multiple comparisons? It’s not that simple. The Annals of Thoracic Surgery, 101(5), 1644–1645. https://doi.org/10.1016/j.athoracsur.2015.11.024
(2010). Using electroencephalography to measure cognitive load. Educational Psychology Review, 22(4), 425–438. https://doi.org/10.1007/s10648-010-9130-y
(2017). Entrained delta oscillations reflect the subjective tracking of time. Communicative & Integrative Biology, 10(5–6), Article
e1349583 . https://doi.org/10.1080/19420889.2017.1349583(1991). Autonomic determinism: The modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological Review, 98(4), Article
459 . https://doi.org/10.1037/0033-295x.98.4.459(2014). Measuring neurophysiological signals in aircraft pilots and car drivers for the assessment of mental workload, fatigue and drowsiness. Neuroscience & Biobehavioral Reviews, 44, 58–75. https://doi.org/10.1016/j.neubiorev.2012.10.003
(2012). Estimating workload using EEG spectral power and ERPs in the n-back task. Journal of Neural Engineering, 9(4), Article
045008 . https://doi.org/10.1088/1741-2560/9/4/045008(2019). Measuring mental workload using physiological measures: A systematic review. Applied Ergonomics, 74, 221–232. https://doi.org/10.1016/j.apergo.2018.08.028
(1993). Attentional and nonattentional forms of sequence learning. Journal of Experimental Psychology: Learning, Memory, and Cognition, 19(1), 189–202. https://doi.org/10.1037/0278-7393.19.1.189
(2011). To choose or to avoid: Age differences in learning from positive and negative feedback. Journal of Cognitive Neuroscience, 23(1), 41–52. https://doi.org/10.1162/jocn.2009.21364
(2008). Better or worse than expected? Aging, learning, and the ERN. Neuropsychologia, 46(2), 521–539. https://doi.org/10.1016/j.neuropsychologia.2007.09.001
(2011). Effects of performance feedback on cardiovascular reactivity and frontal EEG asymmetry. International Journal of Psychophysiology, 81(3), 291–298. https://doi.org/10.1016/j.ijpsycho.2011.07.012
(2005). The influence of task demand and learning on the psychophysiological response. International Journal of Psychophysiology, 56(2), 171–184. https://doi.org/10.1016/j.ijpsycho.2004.11.003
(2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. https://doi.org/10.3758/bf03193146
(2022, November 25). Standard operating procedures for using mixed-effects models. Decision development and psychopathology (D2P2) lab. https://decision-lab.org/resources
(2021). Encoding and consolidation of motor sequence learning in young and older adults. Neurobiology of Learning and Memory, 185, Article
107508 . https://doi.org/10.1016/j.nlm.2021.107508(2003). Neurophysiological measures of cognitive workload during human-computer interaction. Theoretical Issues in Ergonomics Science, 4(1–2), 113–131. https://doi.org/10.1080/14639220210159717
(2000). Changes in memory processing with age. Current Opinion in Neurobiology, 10(2), 224–231. https://doi.org/10.1016/s0959-4388(00)00073-8
(2019). Modulating human memory via entrainment of brain oscillations. Trends in Neurosciences, 42(7), 485–499. https://doi.org/10.1016/j.tins.2019.04.004
(2014). Combining and comparing EEG, peripheral physiology and eye-related measures for the assessment of mental workload. Frontiers in Neuroscience, 8, Article
322 . https://doi.org/10.3389/fnins.2014.00322(2021). Age-related differences in resting-state EEG and allocentric spatial working memory performance. Frontiers in Aging Neuroscience, 13, Article
704362 . https://doi.org/10.3389/fnagi.2021.704362(2013). Neural correlates of the age-related changes in motor sequence learning and motor adaptation in older adults. Frontiers in Human Neuroscience, 7, Article
142 . https://doi.org/10.3389/fnhum.2013.00142(2015). The role of visual processing in motor learning and control: Insights from electroencephalography. Vision Research, 110, 277–285. https://doi.org/10.1016/j.visres.2014.12.024
(2011). Assessment of the cerebral cortex during motor task behaviours in adults: A systematic review of functional near infrared spectroscopy (fNIRS) studies. NeuroImage, 54(4), 2922–2936. https://doi.org/10.1016/j.neuroimage.2010.10.058
(2016). Differences in EEG power in young and mature healthy adults during an incidental/spatial learning task are related to age and execution efficiency. Age, 38(2), Article
37 . https://doi.org/10.1007/s11357-016-9896-z(2009).
Neurophysiological measures of brain activity: Going from the scalp to the brain. Foundations of augmented cognition . In D. D. SchmorrowI. V. EstabrookeM. GrootjenEds., Foundations of augmented cognition: Neuroergonomics and operational neuroscience. FAC 2009. Lecture notes in computer science (Vol. 5638, pp. 488–494). Springer. https://doi.org/10.1007/978-3-642-02812-0_57(2009). The serial reaction time task revisited: A study on motor sequence learning with an arm-reaching task. Experimental Brain Research, 194(1), 143–155. https://doi.org/10.1007/s00221-008-1681-5
(2010). Visual search for real world targets under conditions of high target-background similarity: Exploring training and transfer in younger and older adults. Acta Psychologica, 134(1), 29–39. https://doi.org/10.1016/j.actpsy.2009.12.001
(1987). Attentional requirements of learning: Evidence from performance measures. Cognitive Psychology, 19(1), 1–32. https://doi.org/10.1016/0010-0285(87)90002-8
(2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011, Article
156869 . https://doi.org/10.1155/2011/156869(1998). What’s wrong with Bonferroni adjustments. British Medical Journal, 316(7139), 1236–1238. https://doi.org/10.1136/bmj.316.7139.1236
(2011). Age-related alterations in performance monitoring during and after learning. Neurobiology of Aging, 32(7), 1320–1330. https://doi.org/10.1016/j.neurobiolaging.2009.07.016
(2008). Changes of performance monitoring with learning in older and younger adults. Psychophysiology, 45(4), 559–568. https://doi.org/10.1111/j.1469-8986.2008.00651.x
. (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.r-project.org/
(2002). New visions of the aging mind and brain. Trends in Cognitive Sciences, 6(9), 394–400. https://doi.org/10.1016/s1364-6613(02)01957-5
(1990). No adjustments are needed for multiple comparisons. Epidemiology, 1(1), 43–46. https://doi.org/10.1097/00001648-199001000-00010
(2007). Theta synchronization networks emerge during human object-place memory encoding. Neuroreport, 18(5), 419–424. https://doi.org/10.1097/WNR.0b013e3280586760
(2003). Controlled & automatic processing: Behavior, theory, and biological mechanisms. Cognitive Science, 27(3), 525–559.
(2011). Estimation of mental effort in learning visual search by measuring pupil response. PLoS One, 6(7), Article
5 . https://doi.org/10.1371/journal.pone.0021973(2000). Theta oscillations index human hippocampal activation during a working memory task. Proceedings of the National Academy of Sciences, 97(2), 919–924. https://doi.org/10.1073/pnas.97.2.919
(2019). Non-invasive neurophysiological measures of learning: A meta-analysis. Neuroscience & Biobehavioral Reviews, 99, 59–89. https://doi.org/10.1016/j.neubiorev.2019.02.001
(2020a). Neurophysiological changes in visuomotor sequence learning provide insight in general learning processes: Measures of brain activity, skin conductance, heart rate and respiration. International Journal of Psychophysiology, 151, 40–48. https://doi.org/10.1016/j.ijpsycho.2020.02.015
(2020b). Non-invasive neurophysiology in learning and training: Mechanisms and a SWOT analysis. Frontiers in Neuroscience, 14, Article
589 . https://doi.org/10.3389/fnins.2020.00589(2021). Measures of prefrontal functional near-infrared spectroscopy in visuomotor learning. Experimental Brain Research, 239(4), 1061–1072. https://doi.org/10.1007/s00221-021-06039-2
(2016).
Age-related slowing in response times, causes and consequences . In N. PachanaEd., Encyclopedia of geropsychology. Springer. https://doi.org/10.1007/978-981-287-080-3_211-2(1966). Unobtrusive measures: Nonreactive research in the social sciences. Rand McNally.
(1989). On the development of procedural knowledge. Journal of Experimental Psychology: Learning, Memory and Cognition, 15(6), 1047–1060. https://doi.org/10.1037/0278-7393.15.6.1047
(1994). Factors affecting light-adapted pupil size in normal human-subjects. Investigative Ophthalmology and Visual Science, 35(3), 1132–1137.
(2015). Age-related slowing of response selection and production in a visual choice reaction time task. Frontiers in Human Neuroscience, 9, Article
193 . https://doi.org/10.3389/fnhum.2015.00193(2000). Beta activity: A carrier for visual attention. Acta Neurobiologiae Experimentalis, 60(2), 247–260.



