How Life Before Birth Affects Human Health and What We Can Do About It
In most Western countries, age reckoning starts with “zero,” so that twelve months after birth, a child is considered one year old. In the traditional East Asian age reckoning system, originating in China and still widely used in some East Asian countries such as Korea, newborns start life outside the womb at one year old (becoming two years old on the first day of the subsequent lunar New Year’s day). This not only results in interesting intercultural constellations – a Korean baby born in November 2014 might be one year older than a Swiss baby born in December 2013 – but the traditional East Asian age reckoning also gently reminds us of something else: Life starts to “count” well before birth. In science, however, the relevance of life before birth for our health throughout life has been largely neglected for far too long.
However, during the past decades, numerous studies have pointed out the relevance of (behaviorally relevant) adversities during pregnancy as risk factors for offspring mental disorders, bodily processes, and physical diseases throughout life (e.g., Barker & Thornburg, 2013; Dreier, Andersen, & Berg-Beckhoff, 2014; Entringer et al., 2011; Tegethoff, Greene, Olsen, Schaffner, & Meinlschmidt, 2012; Tegethoff, Olsen, Schaffner, & Meinlschmidt, 2013; Tegethoff, Pryce, & Meinlschmidt, 2009; Tegethoff, Raul, et al., 2011; Van den Bergh, 2011). What has been merged under the term “developmental origins of health and disease” is a highly topical dynamic research field focusing on the fetal period of development, with the aim of elucidating relevant adversities, their costs for offspring development and health, and the biological mechanisms involved in relating the intrauterine environment and long-term results. While the field has already been extensively reviewed (e.g., Beydoun & Saftlas, 2008; Gluckman, Hanson, & Low, 2011; Hertzman, 2012; Koletzko et al., 2014; Luoto, Mottola, & Hilakivi-Clarke, 2013; Tegethoff, 2009), concise overviews of protective factors promoting resilience, and – even more importantly – of interventions aimed at modifying the above-mentioned risk factors are hitherto largely lacking, with a few exceptions (e.g., Koletzko et al., 2012). This special issue of European Psychologist is intended as a contribution toward closing this gap.
It has repeatedly been shown that adversities during pregnancy may impact on the developing fetus (e.g., Dieter et al., 2001; Hansen, Lou, & Olsen, 2000; Huttunen & Niskanen, 1978; Khashan et al., 2008; Tegethoff, Greene, Olsen, Meyer, & Meinlschmidt, 2010a, 2010b). As one prominent example, a study on intrauterine growth of the offspring of women who were pregnant at the time of the 9/11 terrorist attacks in the US indicated that stressful experiences during pregnancy may affect intrauterine processes (Berkowitz et al., 2003). Decades earlier, David Barker and colleagues from Southampton in the UK showed in various studies that low birth weight is related to an increased risk of morbidity and premature mortality (Barker, 2007). Based on these findings, they formulated the “fetal origins of adult disease hypothesis” in the 1980s (later reframed as the “developmental origins of health and disease” (DOHAD) hypothesis), stating that “undernutrition in utero permanently changes the body’s structure, physiology, and metabolism, and leads to coronary heart disease and stroke in adult life” (Barker, 1998, p. 13). They developed this hypothesis based on findings of a strong association between infant mortality rates at the beginning of the 20th century and rates of coronary heart disease and respiratory cancer occurring in the 1960s and 1970s in Norway, England, Wales, and the US (Barker & Osmond, 1986; Buck & Simpson, 1982; Forsdahl, 1977). From these findings, they concluded that adversities very early in life are associated with the risk of disease in adulthood. Epidemiological research around the globe has since extended these initial observations, indicating that a variety of exposures, such as psychosocial stress during pregnancy, are linked to a wide range of adverse health outcomes in the mother during the postpartum period (see Lonstein, Maguire, Meinlschmidt, & Neumann, 2014; Meinlschmidt, Martin, Neumann, & Heinrichs, 2010) and in the offspring (Tegethoff, Greene, Olsen, Schaffner, & Meinlschmidt, 2011).
Notably, research in the field of fetal origins of health and disease is more than an academic issue (Tegethoff, 2009). This topic has recently become a matter of increasing public concern: In 2006, for example, the World Health Organization (WHO) underlined the urgent need to not only scrutinize the long-term effects of early adversities but also to foster optimal fetal development. The report underlines that the global burden of morbidity and mortality resulting from untoward fetal development is substantial, even in developed countries. It further states: “The promotion of optimal fetal development should result in improved outcomes for early and later survival, morbidity and other measures of human capital. This in turn will enhance population social and economic health and well-being” (Word Health Organization, 2006, p. 2). In a similar vein, the Pregnancy and Perinatology Branch of the Center for Developmental Biology and Perinatal Medicine at the National Institute of Child Health and Human Development had previously outlined research priorities for the years 2005–2010, including the identification of determinants of fetal development and the elucidation of consequences of intrauterine adversities, such as maternal stress and substance use during pregnancy, for health throughout life (National Institutes of Health, 2003).
Besides public health professionals, the general population also takes a growing interest in this kind of research, not least because it is – in addition to rare events, such as major catastrophes – primarily common exposures that may hamper fetal development. The high awareness of this topic is reflected in increasing media reporting of the latest research. For example, several major print media covered a study reporting that maternal cell phone use during pregnancy was linked with behavioral difficulties such as emotional and hyperactivity problems in the offspring around the age of school entry (Divan, Kheifets, Obel, & Olsen, 2008). Notably in this regard, headlines such as “Warning: Using a mobile phone while pregnant can seriously damage your baby” (Lean, 2008) occasionally divert from the fact that findings are often merely correlational and should be interpreted with great caution (Divan et al., 2008; Lean, 2008). This anecdote illustrates that with increasing public awareness of research results, the relevance of the issue of risk perception in pregnant women will increase and that – despite the high relevance of the topic – sound knowledge is still limited and much research remains to be done before pregnant women can be provided with clear recommendations on the do’s and don’ts during pregnancy.
In this special issue on how life before birth affects human health and what we can do about it, experts from different countries present state-of-the-field reviews on different aspects of the early phase of life, shifting the focus from basic science to the clinical implications so far maintainable (see Figure 1 ). The articles shed light on (a) factors of resilience and interventions related to very preterm birth, (b) adoption of children growing up in devastating environments as a specific way to intervene in case of early untoward conditions, and (c) a range of adverse exposures and health-related behaviors (i.e., maternal substance use, psychosocial stress, risk perception) during pregnancy, with regard to their consequences for offspring psychological and physiological processes as well as mental and physical health, and provide some evidence-based hints suggesting what we can do about this. 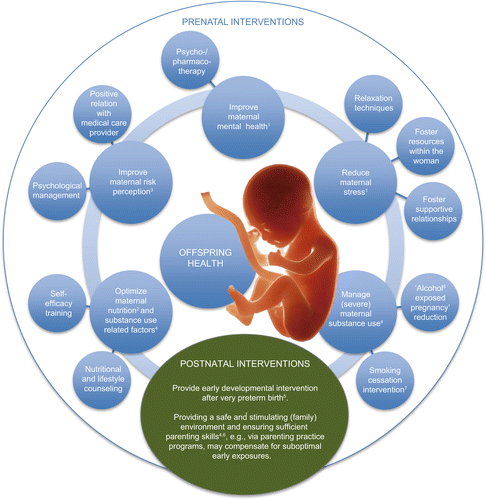
Huizink (2015) elucidates the association between maternal substance use during pregnancy and offspring outcome. She argues for the necessity of putting substance use in a broader context. It is not only substance use during pregnancy itself that may impact on the fetus – factors that lead to substance use during pregnancy also appear to be of relevance, they should be properly acknowledged in research and may be important targets for interventions. The author also presents recommendations for clinical work.
La Marca-Ghaemmaghami and Ehlert (2015) outline how psychosocial stress during pregnancy is related to offspring mental and physical disturbances. They highlight potential mechanisms that may underlie these associations and describe what can be done toward coping with stress during pregnancy. They argue for a need for closer collaboration between science and clinical practice.
Research on long-term consequences of prenatal experiences, for example maternal stress, brings about some specific ethical challenges. For example, what if informing the public about findings puts even more stress on pregnant women by increasing their perception of potential risks for their unborn child? Robinson and colleagues (2015) address such topics, focusing on risk perception during pregnancy that plays an increasing role, given the advances in obstetric management and technology. They outline how mothers most vulnerable to increased risk perception may be identified and describe how findings can assist health care providers to support pregnant women through both high- and low-risk pregnancy and birth.
In the research field of prenatal programming, preterm birth has often been viewed in a very specific way, namely as a proxy for an adverse intrauterine environment (e.g., Tegethoff, Knierzinger, Meyer, & Meinlschmidt, 2013). From a more classical point of view, preterm birth is a major challenge in the field of obstetrics and gynecology. Despite strong efforts to reduce its incidence, preterm birth is still on the rise (US Department of Health and Human Services, National Institutes of Health, & Eunice Kennedy Shriver National Institute of Child Health and Human Development, 2008). Lemola (2015) scrutinizes long-term consequences of very preterm birth and potential mechanisms underlying this link. Furthermore, he sheds light on potential interventions to improve health outcomes after preterm birth and discusses directions for future research.
Last but not least, Kumsta and colleagues (2015) expand the time window of pregnancy to the first years of life. They focus on “adoption” as a specific intervention in the context of reducing harmful early adversities. They report on findings from the English & Romanian Adoptees (ERA) study that follows children who spent their first years of life in extremely depriving Romanian institutions before families in the UK adopted them.
With this special issue, we hope to provide a broad readership with an overview of what is known about the long-term untoward consequences of certain adversities during pregnancy and of what can be done to improve life before birth. At the same time, we would like to encourage scientists in the research field of “long-term effects of early experiences” to further move forward and focus to a greater extent on the promotion of long-term health and quality of life through interventions early in life. In this regard, the reviews provided in this special issue outline some starting points, but they also stress that further efforts to bring this field of research from bench to bed, from the laboratory to the clinic, and from knowledge into action count first and foremost.
Gunther Meinlschmidt, PhD, is a senior lecturer at the Department of Psychology of the University of Basel, Switzerland, and Professor of Psychobiology, Psychosomatics, and Psychotherapy at the Faculty of Medicine of the Ruhr University Bochum, Germany. His research interests include the etiology and therapy of mental and psychosomatic disorders. 
Marion Tegethoff, PhD, is a senior research scientist, senior lecturer, and Ambizione research group leader (SNSF) at the Department of Psychology of the University of Basel, Switzerland. Her research focuses, among others, on the early determinants of mental disorders and physical diseases and on psychobiological therapy approaches. 
References
(2007). The origins of the developmental origins theory. Journal of Internal Medicine, 261, 412–417.
(1986). Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet, 1, 1077–1081.
(2013). The obstetric origins of health for a lifetime. Clinical Obstetrics and Gynecology, 56, 511–519. doi: 10.1097/GRF.0b013e31829cb9ca
(1998). Mothers, babies, and health in later life (2nd ed.). Edinburgh/New York, NY: Churchill Livingstone.
(2003). The World Trade Center disaster and intrauterine growth restriction. JAMA – Journal of the American Medical Association, 290, 595–596.
(2008). Physical and mental health outcomes of prenatal maternal stress in human and animal studies: A review of recent evidence. Paediatric and Perinatal Epidemiology, 22, 438–466.
(1982). Infant diarrhoea and subsequent mortality from heart disease and cancer. Journal of Epidemiology and Community Health, 36, 27–30.
(2001). Maternal depression and increased fetal activity. Journal of Obstetrics and Gynaecology, 21, 468–473.
(2008). Prenatal and postnatal exposure to cell phone use and behavioral problems in children. Epidemiology, 19, 523–529.
(2014). Systematic review and meta-analyses: Fever in pregnancy and health impacts in the offspring. Pediatrics, 133, e674–688. doi: 10.1542/peds.2013-3205
(2011). Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proceedings of the National Academy of Sciences of the USA, 108, E513–518. doi: 10.1073/pnas.1107759108
(1977). Are poor living conditions in childhood and adolescence an important risk factor for arteriosclerotic heart disease? British Journal of Preventive & Social Medicine, 31, 91–95.
(2011). The role of developmental plasticity and epigenetics in human health. Birth Defects Research. Part C, Embryo Today: Reviews, 93, 12–18. doi: 10.1002/bdrc.20198
(2000). Serious life events and congenital malformations: A national study with complete follow-up. Lancet, 356, 875–880.
(2012). Putting the concept of biological embedding in historical perspective. Proceedings of the National Academy of Sciences of the USA, 109, 17160–17167. doi: 10.1073/pnas.1202203109
(2015). Prenatal maternal substance use and offspring outcomes: Overview of recent findings and possible interventions. European Psychologist, 20, 90–101. doi: 10.1027/1016-9040/a000197
(1978). Prenatal loss of father and psychiatric disorders. Archives of General Psychiatry, 35, 429–431.
(2008). Reduced infant birthweight consequent upon maternal exposure to severe life events. Psychosomatic Medicine, 70, 688–694.
(2012). Ernährung in der Schwangerschaft–Teil 2: Handlungsempfehlungen des Netzwerks “Gesund ins Leben–Netzwerk Junge Familie”
[Nutrition in pregnancy–Part 2: Practice recommendations of the Network “Healthy Start Into Life–Young Family Network”] . Deutsche Medizinische Wochenschrift, 137, 1366–1372. doi: 10.1055/s-0032-1305076(2014). The Power of Programming and the EarlyNutrition project: Opportunities for health promotion by nutrition during the first thousand days of life and beyond. Annals of Nutrition & Metabolism, 64, 187–196. doi: 10.1159/000365017
(2015). Psychological consequences of early global deprivation: An overview of findings from the English & Romanian Adoptees Study. European Psychologist, 20, 138–151. doi: 10.1027/1016-9040/a000227
(2015). Stress during pregnancy: Experienced stress, stress hormones, and protective factors. European Psychologist, 20, 102–119. doi: 10.1027/1016-9040/a000195
(2008). Warning: Using a mobile phone while pregnant can seriously damage your baby. The Independent. Retrieved from www.independent.co.uk/life-style/health-and-families/health-news/warning-using-a-mobile-phone-while-pregnant-can-seriously-damage-your-baby-830352.html
(2015). Long-term outcomes of very preterm birth: Mechanisms and interventions. European Psychologist, 20, 128–137. doi: 10.1027/1016-9040/a000207
(2014). Emotion and mood adaptations in the peripartum female: Complementary contributions of GABA and oxytocin. Journal of Neuroendocrinology, 26, 649–664. doi: 10.1111/jne.12188
(2013). Pregnancy and lifestyle: Short- and long-term effects on mother’s and her children’s health. Journal of Pregnancy, 2013, 537526. doi: 10.1155/2013/537526
(2010). Maternal cortisol in late pregnancy and hypothalamic-pituitary-adrenal reactivity to psychosocial stress postpartum in women. Stress, 13, 163–171. doi: 10.3109/10253890903128632
. (2003). Pregnancy and Perinatology Branch strategic plan 2005–2010. Washington, DC: National Institutes of Health.
(2015). Risk perception in pregnancy: Context, consequences, and clinical implications. European Psychologist, 20, 120–127. doi: 10.1027/1016-9040/a000212
(2009). Fetal origins of pediatric disease, Fetoplacental plasticity and intrauterine programming by stress and glucocorticoids. Göttingen, Germany: Cuvillier.
(2010a). Maternal psychosocial adversity during pregnancy is associated with length of gestation and offspring size at birth: Evidence from a population-based cohort study. Psychosomatic Medicine, 72, 419–426. doi: 10.1097/PSY.0b013e3181d2f0b0
(2010b). Maternal psychosocial stress during pregnancy and placenta weight: Evidence from a national cohort study. PLoS One, 5, e14478. doi: 10.1371/journal.pone.0014478
(2011). Stress during pregnancy and offspring pediatric disease: A National Cohort Study. Environmental Health Perspectives, 119, 1647–1652. doi: 10.1289/ehp. 1003253
(2012). Inhaled glucocorticoids during pregnancy and offspring pediatric diseases: A national cohort study. American Journal of Respiratory and Critical Care Medicine, 185, 557–563. doi: 10.1164/rccm.201108-1482OC
(2013). Cortisol awakening response in infants during the first six postnatal months and its relation to birth outcome. Psychoneuroendocrinology, 38, 629–637. doi: 10.1016/j.psyneuen.2012.08.002
(2013). Asthma during pregnancy and clinical outcomes in offspring: A national cohort study. Pediatrics, 132, 483–491. doi: 10.1542/peds.2012-3686
(2009). Effects of intrauterine exposure to synthetic glucocorticoids on fetal, newborn, and infant hypothalamic-pituitary-adrenal axis function in humans: A systematic review. Endocrine Reviews, 30, 753–789. doi: 10.1210/er.2008-0014
(2011). Dehydroepiandrosterone in nails of infants: A potential biomarker of intrauterine responses to maternal stress. Biological Psychology, 87, 414–420. doi: 10.1016/j.biopsycho.2011.05.007
. (2008). Pregnancy and Perinatology Branch (PPB) NICHD: Report to the NACHHD Council September 2008. Bethesda, MD: US Department of Health and Human Services.
(2011). Developmental programming of early brain and behaviour development and mental health: A conceptual framework. Developmental Medicine and Child Neurology, 5, 19–23. doi: 10.1111/j.1469-8749.2011.04057.x
. (2006). Promoting optimal fetal development: Report of a technical consultation. Geneva, Switzerland: World Health Organization.
The authors have no financial relationships relevant to this article to disclose. Gunther Meinlschmidt and Marion Tegethoff receive funding from the Swiss National Science Foundation (to GM: Project No. 100014_135328; to MT: Project No. PZ00P1_137023) and from the Korea Research Foundation within the Global Research Network Program (to GM and MT: Project No. 2013S1A2A2035364). The funding sources had no involvement in the writing and the decision to submit the manuscript for publication.



