Ginkgo biloba Extract EGb 761® in Children with ADHD
Preliminary Findings of an Open Multilevel Dose-Finding Study
Abstract
Objectives: The side effects, nonresponse, and prejudices against conventional pharmacological treatments call for complementary or alternative medical treatments (CAM) for ADHD. One possible treatment, at least for cognitive problems, might be the administration of Ginkgo biloba, though evidence is currently rare. This study tests the clinical efficacy of a Ginkgo biloba special extract (EGb 761®) and its correlation with brain electrical activity in children with ADHD combined type according to DSM-IV. Method: In this open clinical pilot study, EGb 761® was administered to 20 children with ADHD over 3 to 5 weeks. Dosage was increased to a maximum of 240 mg daily if attention problems persisted. Possible drug side effects were assessed using the Side Effect Rating Scale. Efficacy was assessed in a multilevel approach including clinical assessment, quality of life (QoL), as well as performance and preparatory brain-electrical activity evoked during a Continuous Performance Test (Cue-CNV in the CPT). Results: A very low rate of mild adverse effects occurred during the observation period. Following EGb 761® administration, possible improvements in QoL, ADHD core symptoms as well as CPT performance were detected. Improved core symptoms were positively related to elevated CNV amplitude. Conclusion: This preliminary evidence suggests that EGb 761® at a maximal dosage of 240 mg daily might be a clinically useful alternative treatment for children with ADHD, but further evidence is required before firm conclusions can be made.
Fragestellung: Unerwünschte Arzneimittelwirkungen, fehlende Wirksamkeit und Vorurteile gegenüber herkömmlichen medikamentösen Behandlungsformen verlangen nach alternativen medizinischen Behandlungsmöglichkeiten der ADHS. Eine erfolgversprechende, bislang kaum untersuchte Möglichkeit zur Behandlung kognitiver Aspekte ist die Gabe von Ginkgo biloba. Ziel der vorliegenden Studie war die Prüfung klinischer Wirksamkeit und deren Zusammenhang mit hirnelektrischer Aktivität unter der Gabe von Ginkgobiloba-Extrakt EGb 761® bei Kindern mit ADHS vom kombinierten Subtyp nach DSM-IV. Methodik: EGb 761® wurde in einer offenen, klinischen Studie 20 Kindern mit ADHS über 3 bis 5 Wochen verabreicht. Die Dosis wurde bis maximal 240 mg täglich erhöht, solange klinisch relevante Aufmerksamkeitsprobleme bestanden. Klinische Wirksamkeit wurde auf mehreren Ebenen untersucht und beinhaltete klinische Untersuchung, Lebensqualität und Verhaltens- und hirnelektrische Aktivitätsparameter während eines Continuous Performance Tests (Cue-CNV im CPT). Ergebnisse: Im Beobachtungszeitraum traten nur wenige leicht ausgeprägte unerwünschte Wirkungen auf. Nach Gabe von EGb 761® kam es zu möglichen Verbesserung von Lebensqualität, ADHS-Kernsymptomatik und Leistung im CPT. Verbesserungen der Kernsymptomatik waren gleichzeitig mit erhöhter CNV-Amplitude korreliert. Schlussfolgerung: Die Ergebnisse lassen vermuten, dass EGb 761® mit einer Dosis von bis zu 240 mg täglich eine verträgliche und klinisch wirksame alternative Behandlungsmöglichkeit für Kinder mit ADHS darstellt, wenngleich weitere Studien erforderlich sind, um diese vorläufigen Befunde zu stützen.
Introduction
Drug treatment with methylphenidate, amphetamine sulphate, and atomoxetine has been highly recommended and successful in attention deficit/hyperactivity disorder (ADHD) (Banaschewski et al., 2006). However, the side effects, nonresponse, and prejudices against such drugs by some parents demand more tolerable and more acceptable treatments.
Although a lifetime prevalence of herbal therapy of about 20% exists in child psychiatric patients (Cala, Crismon, & Baumgartner, 2003), empirical research concerning complementary/alternative medicine (CAM) for psychiatric disorders in children and adolescents is scarce (Lorenc, Ilan-Clarke, Robinson, & Blair, 2009; Soh & Walter, 2008). Furthermore, the available studies have numerous limitations (Feucht & Patel, 2011). This is all the more important because, first, several clinically relevant adverse reactions were reported in association with some CAMs (about 1%; Jacobsson, Jonsson, Gerden, & Hagg, 2009) and, second, interaction problems may arise when self-administered herbal medicines and prescribed drugs are taken at the same time, specifically because, third, the children’s psychiatrists/pediatricians are usually not aware of such use (Cala et al., 2003). Hence, more empirically based knowledge and experience seem to be necessary, not only in order to test CAM for clinical efficacy and effectiveness, but also because of safety aspects, especially in ADHD.
The standardized Ginkgo biloba extract (EGb 761®1) might be one such possibility, at least inasmuch as related to cognitive problems in ADHD. Although the exact pharmacological mechanism is not fully understood (Nathan, 2000), a number of studies indicate positive effects in adults, particularly in the treatment of cognition in dementia (Birks & Grimley Evans, 2009; Dimpfel, Kler, Kriesl, Lehnfeld, & Keplinger-Dimpfel, 2006; Herrschaft et al., 2012; Itil, Eralp, Tsambis, Itil, & Stein, 1996; Rigney, Kimber, & Hindmarch, 1999; Semlitsch, Anderer, Saletu, Binder, & Decker, 1995; Subhan & Hindmarch, 1984; Weinmann, Roll, Schwarzbach, Vauth, & Willich, 2010), but findings on ADHD are rare (Sarris, Kean, Schweitzer, & Lake, 2011).
Additionally, electroencephalographic studies suggest that Ginkgo biloba has vigilance-enhancing and cognitively activating effects on performance and brain electrical activity (Dimpfel et al., 2006; Itil et al., 1996; Semlitsch et al., 1995).
Since ADHD is characterized by cognitive dysfunctions, the beneficial effects of Ginkgo biloba in this area may also be helpful for treatment. Children, adolescents, and adults with ADHD all show performance deficits in tasks tapping executive functions such as sustained attention, response preparation, and response monitoring. These processes can be investigated with a continuous performance test (CPT), which comprises a cued Go/Nogo test. Patients with ADHD show slower and less homogeneous responses, more omission errors indicating lapses in attention, and false alarms related to impulsivity. The cue stimulus can evoke brain electrical activity with a negative shift of slow frequency located at centro-parietal sites. This contingent negative variation (CNV) is associated with response preparation and time estimation. Diminished CNV amplitude is associated with performance problems and may be a familially driven life-long impairment in ADHD (Albrecht et al., 2012; Doehnert, Brandeis, Straub, Steinhausen, & Drechsler, 2010; McLoughlin et al., 2010). In addition, performance is affected in ADHD when cognitive control is required during the processing of incongruent items, which can be tested with a modified CPT incorporating additional flanker stimuli. Deficits in ADHD are particularly prominent in this more demanding Flanker-CPT (Albrecht et al., 2012; Valko et al., 2009), and it is expected that it is especially sensitive to therapeutic effects.
Recently, Sarris et al. (2011) provided a systematic review of clinical trials on CAM in the treatment of children with ADHD (Sarris et al., 2011): The only available short-term double-blind randomized controlled trial comparing Ginkgo biloba and methylphenidate in two groups (n = 25 each) of children with ADHD revealed that both interventions lead to behavioral improvement; Ginkgo biloba, however, was better tolerated (MPH led to increased reports of headache, insomnia and decreased appetite) but less effective than methylphenidate (both Ginkgo biloba and MPH led to significant improvements in the ADHD Rating Scale of Δd = 0.57 and Δd = 1.39, respectively) (Salehi et al., 2010).
In two open studies, Ginkgo biloba extracts showed promising preliminary results for treating the core symptoms of ADHD; however, the interpretation of these results is limited by the small sample size in one study (N = 6, Niederhofer, 2010)) and coadministration with American ginseng extract as another CAM in the other study (Lyon et al., 2001). Thus, further research should be undertaken in order to overcome these inconsistent results.
Since the efficacy of treatments for ADHD should be measured at different levels of functioning (reflecting ecological validity), this multilevel dose-finding pilot study was conducted not only to describe the behaviorally clinical effects and the safety of Ginkgo biloba EGb 761® in children with ADHD, but also to assess its effects at other levels including performance during a sustained attention task – while registering in parallel the brain electrical activity during response preparation and testing whether both levels are both statistically related to each other. In contrast to other studies with Ginkgo biloba in children, not only a broader psychopathological profile as well as quality of life, but also brain electrical activity were taken into consideration in order to assess treatment effects on different levels, which may counteract the limitations of an otherwise open pilot study
Methods
Sample
From the files of the special outpatient unit on ADHD of the Department of Child and Adolescent Psychiatry at the University of Göttingen (Germany), 24 children with clinical diagnosis of ADHD combined type were recruited among the consecutive referrals to our outpatient clinic. Four of the children were excluded because of missing (N = 1) or withdrawn consent (N = 2) or susceptibility of a secondary disorder (N = 1).
Thus, altogether 20 children (75% boys) diagnosed with ADHD combined type according to DSM-IV who did not tolerate or were unwilling to take methylphenidate were included in the study. The children were aged 6 to 13 years (mean 8.2 ± 1.6 years) with a mean body height of 134 ± 9.2 cm and a mean weight of 32.0 ± 9.7 kg. All had a total IQ above 80 and went to a regular school. Exclusion criteria were pharmacological treatment within the last 2 weeks, seizure disorder, and other disorders that may mimic ADHD (such as disruptive behavior, anxiety, or depression) as well as other medical problems such as hypertension. The assessments within the framework of the study were done by board-certified child and adolescent psychiatrists using clinical interviewing along DSM-IV and standardized questionnaires with children and parents, followed by neurophysiological testing of the children in the departments EEG laboratory. The protocol was reviewed and approved by the local ethics committee. The principles of Good Clinical Practice and the Declaration of Helsinki were adhered to. Written informed consent of parents and children (for the latter at least assent) was given.
Study Design
In an open single-center study, participants received Ginkgo biloba EGb 761® tablets over a period of 3 to 5 weeks. EGb 761® is a dry extract from Ginkgo biloba leaves (35–67:1), extraction solvent: acetone 60% (w/w). The extract is adjusted to 22.0 – 27.0% ginkgo flavonoids calculated as ginkgo flavone glycosides and 5.0 – 7.0% terpene lactones consisting of 2.8 – 3.4% ginkgolides A, B, C and 2.6 – 3.2% bilobalide, and contains less than 5 ppm ginkgolic acids. The initial dose of 40 mg twice daily was successively increased to 60 and 120 mg twice daily depending on efficacy and tolerability at weekly study visits. The thus determined effective dose was then administered for 3 weeks. Dosage was increased if scores on the primary outcome measure Attention Problems was above 1, indicating clinically relevant difficulties. Figure 1 presents the study protocol. 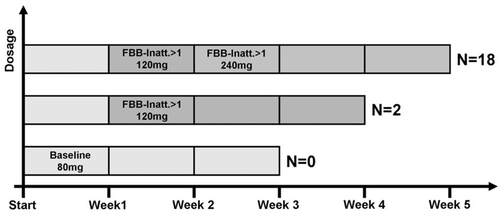
Possible side effects were rated by parents and children using the Side Effects Rating Scale (SERS-D) (Lingjaerde, Ahlfors, Bech, Dencker, & Elgen, 1987).
Behavior Ratings
Primary Outcome Variable
Any changes in parents’ assessment of their children’s attentiveness between start and end of therapy were obtained using the questionnaire FBB-HKS (part of the Diagnostic System of Mental Disorders in Children and Adolescents [DISYPS-KJ], a German DSM-IV-oriented rating scale for ADHD problems; item: severity of attentive problems; Döpfner & Lehmkuhl, 2000; Gortz-Dorten & Döpfner, 2009).
Secondary Outcome Variables
Any changes in parents’ assessment of their children’s hyperactivity and impulsiveness as well as aggressive behaviors were obtained with the DISYPS-KJ FBB-HKS and FBB-SSV, respectively (Döpfner & Lehmkuhl, 2000). Changes in strains on family life were assessed by the FaBel (Ravens-Sieberer et al., 2001); and changes in the children’s perceived quality of life were obtained with the KINDL questionnaire (Ravens-Sieberer & Bullinger, 1998). The general psychopathological profile was assessed using the German version of the children’s strengths and difficulties questionnaire SDQ-D (Goodman, 1997; Woerner, Becker, & Rothenberger, 2004).
Continuous Performance Test
The Continuous Performance Test (CPT; see Figure 2) consists of a sequence of bold black letters presented with a stimulus onset asynchrony of 1650 ms at the center of a 17" CRT monitor against light gray background for 150 ms each, subtending a viewing angle of 0.6° horizontally and 0.8° vertically. A response is required only if the cue (the letter “O,” 20% of all trials) is followed by a target (the letter “X,” 10% of all trials). The impact of additional need for cognitive control throughout all trials was assessed with a Standard-CPT comprising only the central stimuli and a Flanker-CPT with additional incompatible flanker-letters (subtending approx. 2.0° viewing angle vertically). More details can be found elsewhere (Albrecht et al., 2012; Doehnert et al., 2008). 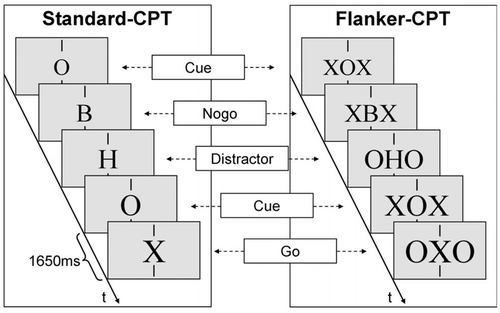
Electrophysiological Recording and Processing
The EEG was registered from 27 Ag/AgCl electrodes according to an extended 10–20 montage including FCz as recording reference. Additional electrodes placed above and below the right eye and at the other canthi to monitor the electroocculogram (EOG) were recorded using BrainAmp amplifier (Brain Products, Munich, Germany). A ground electrode was placed on the forehead, and the sampling rate was set to 500 Hz with recording filters 0.016 to 100 Hz and a 50 Hz notch. Impedances were kept below 10 kΩ.
Offline processing included downsampling to 256 Hz, re-referencing to the average and filtering with 0.1 to 30 Hz/24 db/oct Butterworth filters. Ocular artifacts were corrected on the unsegmented data using a linear regression method (Gratton, Coles, & Donchin, 1983). If the amplitude of any electrode exceeded ±100μV, a section –100 to +800 ms was excluded from further analyses. Segments –125 to 1875 ms around cue-onset without performance errors were averaged, and the contingent negative variation (CNV) was assessed as the mean amplitude 1200 to 1650 ms. All averages contained at least 15 segments.
Analyses
Changes in behavioral rating scales (including the SERS-D) from the pretreatment to the posttreatment assessment were analyzed with the one-sided nonparametric Wilcoxon sign rank test. The CPT data were analyzed with a parametric analysis of variance ANOVA including within-subjects factors Date (pretreatment vs. posttreatment), Flanker (Standard-CPT vs. Flanker-CPT), and for the Cue-CNV also factor Electrode (Cz vs. Pz).
The relationship between ADHD total score symptom severity ratings by parents and the improved CPT parameters was explored using percentage-standardized changes from the preassessment to postassessment (with the preassessment measurement set to 100% for each subject). These parameters were entered into a principal component analysis. It is expected that improvements in FBB-HKS total score (indicated by positive percentage changes) are correlated with improvements in response speed (negative percentages indicating improvements) and improved Cue-CNV (positive percentages indicate improvements, e.g., reduced parental ratings of ADHD severity, faster response speed and lower error rates as well as elevated mean CNV amplitudes in the post assessment).
Statistical power analyses were calculated using G*Power 3 (Faul, Erdfelder, Lang, & Buchner, 2007). With conventional parameters (α set to .05 two-tailed with a power of 1–β = .75), the available full analysis set of 20 participants allows the detection of changes in the psychopathology ratings with an effect size of d = 0.64 using nonparametric Wilcoxon signed rank tests, and changes in the Cue-CNV tested parametrically using analyses of variance in the available sample of N = 13 with effect size of d = 0.8, (or d = 0.68 if also trends with α < .1 are considered).
Results
Safety Data
For two children, 60 mg EGb 761® b.i.d. proved to be the effective dose, whereas all other 18 children received 120 mg b.i.d. as effective dose for 3 weeks. The 20 patients included in the study were treated with EGb 761® over a total period of 703 days. During this period three mild adverse events were observed in three patients. Of these, eosinophilia and allergic dermatitis were unrelated to the administration of EGb 761®. Prolonged thrombin time was unlikely related with study medication intake. Altogether, the incidence of adverse events was 0.004 per observation day, and no serious adverse events occurred under EGb 761®. The ratings of both parents and children of the SERS-D indicated lower burden from symptoms typically encountered as side effects with psychotropic drugs (both p = .05, see Table 1).

Behavior Ratings and Quality of Life
After therapy with EGb 761®, significant improvements were found for the parents’ assessment of their children’s attentiveness (FBB-HKS attention problems, Δd = 0.78, p < .01). Furthermore, severity of the FBB-HKS items hyperactivity, impulsivity, and the total score for symptom severity were decreased significantly (all Δd > 0.61, p < .01, Table 1).
The general psychopathological profile obtained with parent-rated SDQ revealed significant improvement regarding Prosocial Behavior (p < .01) while Peer Problems, Hyperactivity, Emotional Problems, and Conduct Problems remained nearly unchanged (all p > .14, see Table 1). Moreover, improvements were also found in the FBB-SSV Oppositional-Aggressive symptomatology ratings (p < .01) while Dissocial-Aggressive symptoms showed probably a floor-effect with no change (p = .25). Strains on family life (FaBel) also improved (p < .01), but the children’s self-reported quality of life measured with the KINDL remained unchanged (p = .72).
Continuous Performance Test
Due to insufficient performance (less than 50% hit-rate or more than 25% commission errors in cued Nogo trials, N = 4) or an insufficient number of artifact-free Cue-trial EEG-sweeps (less than 15 sweeps, N = 4), a total of 7 datasets were excluded from the event-related potentials (ERP) analysis. This total dropout rate of 35% is due to a mean dropout rate of ~10% for each of the four CPT assessments conducted, which is similar to other studies (Albrecht et al., 2012; Banaschewski et al., 2003). The excluded subjects did not differ in age (F(1, 18) = 2.0, p = .18), nor FBB-HKS total scores from preassessment or postassessment or percentage change from the 13 subjects included in the ERP analysis (all F(1, 18) < 1, p > .47).
Performance
Reaction times were slower for the Flanker-CPT compared to the Standard-CPT (Flanker: F(1, 12) = 13.1, p < .01, part η2 = 0.52), but they also showed some tendency toward improvement (p = .06 posthoc), which was not present in the Standard-CPT (Time: F(1, 12) = 2.7 p = .13, part η2 = 0.19; Flanker*Time: F(1, 12) = 1.9 p = .19, part η2 = 0.14, see Figure 3a). 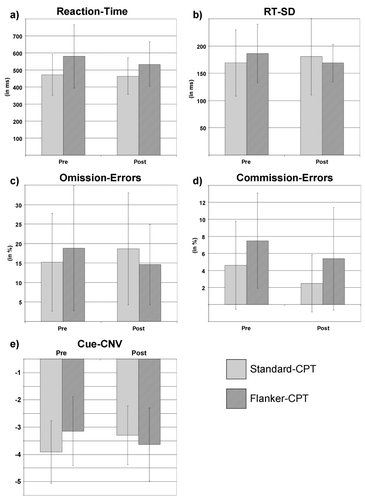
Reaction-time variability was similar for both tasks in both assessments (all F(1, 12) < 2.0 p > .19, d < 0.39, Figure 3b).
Omission error rate showed a trend toward an interaction effect Flanker*Time (F(1, 12) = 3.3 p = .09, d = 0.51), which was driven by increased omission error rate in the Standard-CPT, but rather decreased omission error rate in the Flanker-CPT (Figure 3c). Commission errors were more frequent in the Flanker-CPT (F(1, 12) = 10.8 p = .01, d = 1.05); other effects were not present (see Figure 3d).
Event-Related Brain Activity: Preparatory Cue-CNV
The Cue-CNV mean amplitude showed a strong trend toward an interaction effect Flanker*Time (F(1, 12) = 4.0 p = .07, d = 0.55), driven by a decreased Cue-CNV in the Standard-CPT (p = .05) and a trend toward increased CNV in the Flanker-CPT (p = .1 one-tailed). All other main and interaction effects were not significant (all F(1, 12) < 1.2, p > .31, part η2 < 0.09, see Figure 3d and Figure 4). 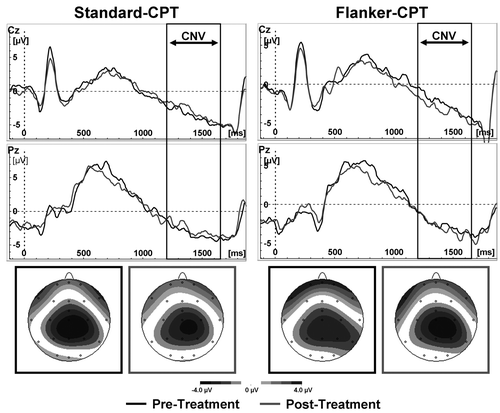
Relation of Changes in ADHD Symptom Severity with Flanker-Continuous Performance Test Parameters
Since improvements were present in FBB-HKS total score and Flanker-CPT reaction time, omission error rate and as a tendency also for mean Cue CNV amplitude, the percent-standardized changes from the preassessment to postassessment (positive values indicating improvements, e.g., shorter RT or more negative CNV at the post assessment) as well as age at preassessment were explored in a principal component analysis. A Scree test favored a two-component solution, which explained 72% of the total variance: improvements in response speed and omission errors as well as the patients age loaded particularly on the first component (which explained 39% variance). Improvements in ADHD total symptom rating, Flanker-CPT Cue CNV mean amplitude showed high loadings on the second component (33% explained variance, see Figure 5 for Varimax-rotated component loadings). 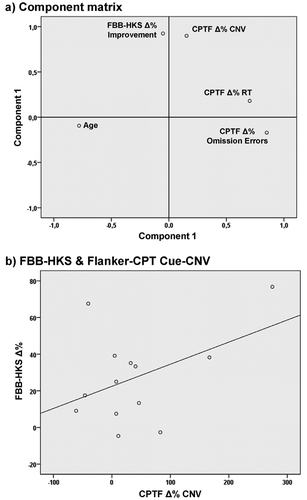
Discussion
The main finding of this pilot CAM-study with EGb 761® was that improvements of ADHD symptomatology were positively related to elevated CNV amplitude, which may yield interrelated effects at different assessment levels and thus may present some preliminary evidence for the efficacy of Ginkgo biloba at a dosage level of up to 240 mg daily.
Concerning safety aspects, the data indicate that EGb 761® was well tolerated by the patients. The incidence of adverse events with a rate of 0.004 per observation day was low compared to common pharmacological treatments such as methylphenidate (e.g., see Döpfner, Gortz-Dorten, Breuer, & Rothenberger, 2011). Administration of EGb 761® revealed improvements at the level of behavioral ratings obtained from parents for the primary outcome variables. Significant improvements were found for attention problems. Similarly, the secondary outcome variables hyperactivity and impulsiveness as well as some oppositional defiance/conduct disorder (ODD/CD) problems also showed improvements. This is roughly in line with previous findings in ADHD drug studies.
However, no improvements were found in the broad-range psychopathological profile obtained with the SDQ. The SDQ reflects several strengths and difficulties of children whereas, the FBB-HKS and FBB-SSV ratings are more disorder specific. This specificity may indicate that improvements detected by the FBB ratings may go beyond general social desirability or unspecific expectancies or bias by the parents who were aware of the (desired) treatment with Ginkgo biloba extract and might have been biased toward positive effects. It has to be discussed how far parents may well expect also specific improvements following the treatment. Moreover, it remains open whether improvements in attention problems were also present during structured school situations, which would have require additional teacher ratings, lacking in this pilot study.
Furthermore, parents might expect little harm when giving CAM to their children, which might be reflected in the lower ratings of side effects after treatment. Moreover, there are hints that the decision to use CAM treatment in children highly depends on the parents’ attitude (probably positive expectations for treatment outcome and/or safety) toward CAM treatment options (Lorenc et al., 2009). Thus, it is ethically imperative to carefully evaluate potential risks of CAMs in clinical studies and to inform the parents about the results in order to prevent a misleading awareness of the risk-benefit ratio.
Quality of life as measured by the parent-rated family burden FaBel total score and the child-rated KINDL showed mixed results: While parents reported significant improvement in the FaBel, the change in the children’s KINDL total score did not reach significance. This may be a power issue, since the magnitude in KINDL change was similar to a recent evaluation study of atomoxetine, which was significant in a larger sample (Wehmeier et al., 2011).
The above-mentioned subjective effects and the related problems for interpretation may not be present at the experimental level of this study, which reflects more objectively the child’s behavior. Findings at this level of description may counteract the missing placebo-arm and help to understand problems associated with ADHD in the framework of neuropsychological constructs. As expected, presentation of additional incompatible flanker stimuli led to generally slower responses, probably because of additional time needed for cognitive control. Under EGb 761® we detected elevated response speed in the Flanker-CPT, which is particularly sensitive to performance problems in ADHD (Albrecht et al., 2012). Reaction-time variability as well as accuracy were not improved. Hence, the faster responses in the second assessment specific for the Flanker-CPT were probably not an effect of an optimized speed-accuracy tradeoff, but rather may reflect a performance improvement per se. However, this may also be influenced by a retest effect which we did not control for.
The third assessment level was evoked brain activity. Here, we focused on an electrophysiological correlate of response preparation. The analyzed CNV mean amplitude is of particular importance in ADHD research. It reflects preparation and time estimation as a proximal biological factor associated with the disorder, and its reduced amplitude may be a life-long endophenotypic impairment in ADHD (Albrecht et al., 2012; Doehnert, Brandeis, Imhof, Drechsler, & Steinhausen, 2010; McLoughlin et al., 2010). Moreover, slow cortical potentials are also a target for neurofeedback training in ADHD (Gevensleben, Rothenberger, Moll, & Heinrich, 2012; Heinrich, Gevensleben, Freisleder, Moll, & Rothenberger, 2004), which highlights the important functional relevance it may have in ADHD. In the current study, the CNV amplitude was increased in the second assessment following administration of EGb 761® in the Flanker-CPT, but decreased in the Standard-CPT. While this disordinal interaction may be partly explained by possible differential motivation effects (that may have led also to a lack of performance improvement in the Standard-CPT), the Flanker-CPT revealed a relative increase in CNV amplitude as a central electrophysiological parameter of preparation-related brain activity and increased response speed in the following Go trial. However, it remains open whether this was influenced by retest effects, as discussed for performance data.
Despite potential alternative explanations for improvements of the three interrelated assessment levels studied, a correlation of changes in the core outcome parameters between these levels would highlight that there may be a common cause for all these effects. To test this assumption, we conducted a principal component analysis between changes from preassessment to the postassessment of the core behavioral rating parameter of ADHD symptomatology, Flanker-CPT performance as well as CNV amplitude. In order to differentiate spurious relationships due to common developmental effects, also the age of the participants was entered. The Scree test favored a two-component solution, which explained almost 3/4 of the total variance of the five variables. Improvements in Flanker-CPT performance as well as age loaded on the first component – and may thus reflect insight effects in task performance strategy. The second component comprises changes in FBB-HKS ADHD symptomatology at the level of behavioral ratings and changes in preparation-related brain activity (CNV). As these two parameters are susceptible to different limitations, their correlation may index a common cause for these improvements, not compromised by developmental trends.
Limitations
This open pilot trial has several limitations, which allow us to speak only of a positive tendency in relation to the possible clinical efficacy of EGb 761® in children with ADHD. Hence, the sample was quite small, and a placebo control group and randomization were missing. There was no blinding and no teacher report, and the observational period was rather short. On the other hand, the present results seem to encourage setting up a randomized controlled trial.
Conclusion
The current short-term open pilot study showed that Ginkgo biloba special extract (EGb 761®) seems to be a well-tolerated complementary or alternative medicine for treating childhood ADHD in patients who do not tolerate or are not willing to take methylphenidate. Treatment with Ginkgo biloba EGb 761® over a total summarized period of more than 700 observation days revealed an incidence rate of 0.004 adverse events per observation day without any serious side effects. Following administration, interrelated improvements on behavioral ratings of ADHD symptoms and electrical brain activity related to response preparation were detected in this open study.
Taken together, the current study provides some preliminary evidence that Ginkgo biloba EGb 761® seems to be well tolerated in the short term and may be a clinically useful treatment for children with ADHD. Double-blind randomized controlled trials are required to clarify the value of the presented data.
Clinical Significance
The trends of this preliminary open study may suggest that Ginkgo biloba special extract (EGb 761®) might be considered as a complementary or alternative medicine for treating children with ADHD. A dosage of 60 to 120 mg twice daily seems to be adequate. But further studies on dose-finding, clinical efficacy and safety on long-term observations are required before firm conclusions can be drawn.
Conflicts of interest
Uebel-von Sandersleben, H.: Speaker’s Bureau: Lilly, MEDICE, Janssen-Cilag, Shire, Novartis Rothenberger, A.: Advisory Board and Speakers Bureau: Lilly, Shire, Medice, Novartis. Research support: Shire, German Research Society, Schwabe; Travel Support: Shire; Educational grant: Shire; Consultant: UCB/Shire; Lilly Albrecht, B.: None Rothenberger, L. G.: None Klement, S.: Employee of Dr. Willmar Schwabe GmbH & Co. KG Bock, N.: None
1EGb 761® is the active substance of Tebonin® (Dr. Willmar Schwabe GmbH & Co. KG; Karlsruhe, Germany).
References
(2012). Familiality of neural preparation and response control in childhood attention deficit-hyperactivity disorder. Psychological Medicine, 43, 1997–2011.
(2003). Association of ADHD and conduct disorder: Brain electrical evidence for the existence of a distinct subtype. Journal of Child Psychology and Psychiatry and Allied Disciplines, 44, 356–376.
(2006). Long-acting medications for the hyperkinetic disorders. A systematic review and European treatment guideline. European Child and Adolescent Psychiatry, 15, 476–495.
(2009). Ginkgo biloba for cognitive impairment and dementia. Cochrane Database of Systematic Reviews, CD003120.
(2003). A survey of herbal use in children with attention-deficit-hyperactivity disorder or depression. Pharmacotherapy, 23, 222–230.
(2006). Neurophysiological characterization of a functionally active drink containing extracts of ginkgo and ginseng by source density analysis of the human EEG. Nutritional Neuroscience, 9, 213–224.
(2010). Mapping attention-deficit/hyperactivity disorder from childhood to adolescence: No neurophysiologic evidence for a developmental lag of attention but some for inhibition. Biological Psychiatry, 67, 608–616.
(2008). Slow cortical potential neurofeedback in attention deficit hyperactivity disorder: Is there neurophysiological evidence for specific effects? Journal of Neural Transmission, 115, 1445–1456.
(2011). An observational study of once-daily modified-release methylphenidate in ADHD: Effectiveness on symptoms and impairment, and safety. European Child and Adolescent Psychiatry, 20(Suppl 2), 243–255.
(2000). Diagnostik-System für Psychische Störungen im Kindes- und Jugendalter nach ICD-10 und DSM-IV (DISYPS-KJ) (2nd ed.). Bern: Huber.
(2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191.
(2011). Herbal medicines in pediatric neuropsychiatry. Pediatric Clinics of North America, 58, 33–54.
(2012). Neurofeedback in children with ADHD: Validation and challenges. Expert Review of Neurotherapeutics, 12, 447–460.
(1997). The Strengths and Difficulties Questionnaire: A research note. Journal of Child Psychology and Psychiatry and Allied Disciplines, 38, 581–586.
(2009). Aufmerksamkeitsdefizit-/Hyperaktivitätsstörungen von Kindern und Jugendlichen im Elternurteil – Eine Analyse an einer Feldstichprobe mit dem Diagnostik-System DISYPS – II [
Attention deficit/hyperactive disorders in children and adolescents as assessed by parents ]. Zeitschrift fur Kinder- und Jugendpsychiatrie und Psychotherapie, 37, 183–194.(1983). A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55, 468–484.
(2004). Training of slow cortical potentials in attention-deficit/hyperactivity disorder: Evidence for positive behavioral and neurophysiological effects. Biological Psychiatry, 55, 772–775.
(2012). Ginkgo biloba extract EGb 761(R) in dementia with neuropsychiatric features: A randomised, placebo-controlled trial to confirm the efficacy and safety of a daily dose of 240 mg. Journal of Psychiatric Research, 46, 716–723.
(1996). Central nervous system effects of ginkgo biloba, a plant extract. American Journal of Therapeutics, 3, 63–73.
(2009). Spontaneously reported adverse reactions in association with complementary and alternative medicine substances in Sweden. Pharmacoepidemiology Drug Safety, 18, 1039–1047.
(1987). The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatrica Scandinavica, 334 (Suppl), 1–100.
(2009). How parents choose to use CAM: a systematic review of theoretical models. BMC Complement Altern Med, 9, 9.
(2001). Effect of the herbal extract combination Panax quinquefolium and Ginkgo biloba on attention-deficit hyperactivity disorder: A pilot study. Journal of Psychiatry and Neuroscience, 26, 221–228.
(2010). Electrophysiological evidence for abnormal preparatory states and inhibitory processing in adult ADHD. Behavioral and Brain Functions, 6, 66.
(2000). Can the cognitive enhancing effects of ginkgo biloba be explained by its pharmacology? Medical Hypotheses, 55, 491–493.
(2010). Ginkgo biloba treating patients with attention-deficit disorder. Phytotherapy Research, 24, 26–27.
(1998). Assessing health-related quality of life in chronically ill children with the German KINDL: First psychometric and content analytical results. Quality of Life Research, 7, 399–407.
(2001). Der Familien-Belastungs-Fragebogen (FaBel-Fragebogen) – Testung und Validierung der deutschen Version der “Impact on Family Scale” bei Familien mit behinderten Kindern [
The testing and validation of the German version of the Impact on Family Scale in families with children with disabilities ]. Psychotherapie, Psychosomatik, Medizinische Psychologie, 51, 384–393.(1999). The effects of acute doses of standardized Ginkgo biloba extract on memory and psychomotor performance in volunteers. Phytotherapy Research, 13, 408–415.
(2010). Ginkgo biloba for attention-deficit/hyperactivity disorder in children and adolescents: A double-blind, randomized controlled trial. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 34, 76–80.
(2011). Complementary medicines (herbal and nutritional products) in the treatment of Attention Deficit Hyperactivity Disorder (ADHD): A systematic review of the evidence. Complementary Therapies in Medicine, 19, 216–227.
(1995). Cognitive psychophysiology in nootropic drug research: Effects of Ginkgo biloba on event-related potentials (P300) in age-associated memory impairment. Pharmacopsychiatry, 28, 134–142.
(2008). Complementary medicine for psychiatric disorders in children and adolescents. Current Opinion in Psychiatry, 21, 350–355.
(1984). The psychopharmacological effects of Ginkgo biloba extract in normal healthy volunteers. International Journal of Clinical Pharmacology Research, 4, 89–93.
(2009). Differences in neurophysiological markers of inhibitory and temporal processing deficits in children and adults with ADHD. Journal of Psychophysiology, 23, 235–246.
(2011). Effect of atomoxetine on quality of life and family burden: results from a randomized, placebo-controlled, double-blind study in children and adolescents with ADHD and comorbid oppositional defiant or conduct disorder. Quality of Life Research, 20, 691–702.
(2010). Effects of Ginkgo biloba in dementia: Systematic review and meta-analysis. BMC Geriatrics, 10, 14.
(2004). Normative data and scale properties of the German parent SDQ. European Child and Adolescent Psychiatry, 13(Suppl 2), II3–33710.
The study was sponsored by Dr. Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany



