The coronavirus disease (COVID-19) – A supportive approach with selected micronutrients
Abstract
Abstract. Worldwide the pandemic of COVID-19 spreads rapidly and has had an enormous public health impact with substantial morbidity and mortality especially in high-risk groups, such as older people and patients with comorbidities like diabetes, dementia or cancer. In the absence of a vaccine against COVID-19 there is an urgent need to find supportive therapies that can stabilize the immune system and can help to deal with the infection, especially for vulnerable groups such as the elderly. This is especially relevant for our geriatric institutions and nursing homes. A major potential contributing factor for elderly is due to their high incidence of malnutrition: up to 80% among the hospitalized elderly. Malnutrition results when adequate macronutrients and micronutrients are lacking in the diet. Often missing in public health discussions around preventing and treating COVID-19 patients are nutritional strategies to support optimal function of their immune system. This is surprising, given the importance that nutrients play a significant role for immune function. Several micronutrients, such as vitamin D, retinol, vitamin C, selenium and zinc are of special importance supporting both the adaptive and innate immune systems. As suboptimal status or deficiencies in these immune-relevant micronutrients impair immune function and reduces the resistance to infections, micronutrient deficiencies should therefore be corrected as soon as possible, especially in the elderly and other vulnerable groups. According to epidemiological, experimental and observational studies, some case reports and a few intervention studies the supplementation of vitamin D and/or zinc are promising. The multiple anti-inflammatory and immunomodulatory effects of Vitamin D could explain its protective role against immune hyper reaction and cytokine storm in patients with severe COVID-19. A randomized, placebo-controlled intervention study even shows that high dose vitamin D supplementation promotes viral clearance in asymptomatic and mildly symptomatic SARS-CoV-2 positive individuals. Besides, the data of a recent prospective study with COVID-19 patients reveal that a significant number of them were zinc deficient. The zinc deficient patients had more complications and the deficiency was associated with a prolonged hospital stay and increased mortality. Thus, immune-relevant micronutrients may help to increase the physiological resilience against COVID-19.
Introduction
At the end of 2019, a coronavirus pandemic started in the city of Wuhan in China’s Hubei province. This new coronavirus has been designated severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) and the pulmonary disease it causes is known as coronavirus disease-2019 (COVID-19). The clinical course of the disease caused by SARS-CoV-2 varies considerably [1, 2]. The successful fight against the coronavirus pandemic will be decided in our geriatric institutions and nursing homes. According to the World Health Organisation (WHO) 40% of all cases will experience mild disease, 40% will experience moderate disease including pneumonia, 15% will experience severe disease, and 5% will have critical disease that rapidly progresses to acute respiratory failure and death. Among all patients, the risk for severe illness from COVID-19 increases with age and comorbidities such as cancer, diabetes, cerebrovascular conditions (e.g. dementia) and cardiovascular diseases, with older adults at highest risk. The early death cases of COVID-19 outbreak occurred primarily in elderly people, possibly due to either a weak immune system that permits faster progression of the viral infection or due to the inability to modulate the immune system in response to the viral infection resulting in a cytokine storm [3, 4].
COVID-19 has spread quickly to all corners of the world, and its capacity for explosive spread has overwhelmed even the most resilient health systems. For many people their daily lives haves been profoundly changed, economies have fallen into recession, and many of the traditional economic, social and public health safety nets that people relied on have been put under unprecedented strain. Worldwide the governments and health policies are taking drastic measures to try and slow the spread of the virus [2, 3]. Apart from appropriate hygiene measures, social and physical distancing measures, corona apps and movement restrictions, such as lock downs there is currently little in media (e.g. TV, radio) or in public health discussions around immunity and infection as it relates to nutritional strategies to support optimal function of the immune system. This is astonishing because of the well-established evidence that micronutrients have for the integrity of immune system. Several micronutrients are essential for innate and adaptive immunity, especially vitamins D, A, C and minerals such as selenium and zinc [4–7].
During the first critical minutes of exposure to a new pathogen, the immune response relies on the innate immune system to protect from infection. The innate immune system is mainly composed by biochemical barriers such as mucous membranes (e.g. respiratory tract, gastrotintestinal tract) and an unspecific cellular response mediated by dendritic cells, monocytes, neutrophils and natural killer cells. The adaptive immune system, also referred as the acquired immune system, involves an antigen-specific response mediated by T and B lymphocytes that is activated by the exposure to a pathogen. Both, the innate and adaptive immune system works together to eliminate a pathogen. Older people (aged 65 years and older) experience commonly a dysfunction of the innate and adaptive immunity that makes them less able to respond to immune challenges. With increasing age, a loss of lymphoid tissue can be observed, particularly in the thymus, and the ability to respond to pathogens is impaired. Nutritional deficiencies of micronutrients, especially vitamin D and zinc may be responsible for the high incidence of infectious diseases related to age [5–7].
Immune-relevant micronutrients collectively function to support the integrity of physical barriers, the growth, differentiation and motility of innate cells, the phagocytic activities of immune cells including macrophages, monocytes and neutrophils, the production of antimicrobial peptides, and the promotion of and the recovery from inflammation (e.g. antioxidant activity, cytokine production). Deficiencies or suboptimal status in micronutrients negatively affect immune function and can decrease resistance to infection [8–11]. Severity and mortality risk of SARS-CoV-2 infection or COVID-19 disease have been associated with age. For instance, a recent pilot study of a North American Community Hospital Intensive Care Unit found low serum levels of 25-hydroxyvitamin D and vitamin C in most of the critically ill COVID-19 ICU patients. Older age and low vitamin C levels appeared to be co-dependent risk factors for mortality [12].
The overproduction of reactive oxygen species (ROS) and a deprived antioxidant system is associated not only with the process of aging but it plays a major role in the pathogenesis and progression of respiratory tract infections such as influenza and SARS-CoV-2 infection. The NLRP3 inflammasome consists of nucleotide-binding domain leucine-rich repeat and pyrin domain containing receptor 3 (NLRP3), apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and procaspase-1. It is a multimeric protein complex that initiates and promotes inflammation by triggering the release of pro-inflammatory cytokines such as IL-1β and IL-18. Reactive oxygen species (ROS) are involved as direct or indirect NLRP3 inflammasome activators in virus replication and inflammation. Thereby the use of antioxidants, such as vitamin C and selenium and anti-inflammatory agents such as vitamin D and zinc could help the recovery of patients with COVID-19 [13–15]. Although not discussed in this review other potentially immune-relevant nutrients which are required to maintain proper immune function, including amino acids (e.g. glutathione, glutamine), antioxidants (e.g. vitamin E), phytochemicals (e.g. quercetin), omega-3 fatty acids or iron should be mentioned at this point.
Immunity and nutritional status
The first line of host defense is called the innate immune system that consists of chemical, physical, and cellular defenses against pathogens. Its main purpose is to prevent immediately the spread and movement of foreign pathogens throughout the body. For example, it contains physical barriers, such as epithelial cell layers that express tight cell-cell contacts and mucus layers that line the epithelium in the respiratory, gastrointestinal and urogenital tract. The second line of defense is called adaptive immune system. The adaptive immune response becomes prominent after several days, as antigen-specific T and B cells have undergone clonal expansion. The adaptive and innate immune systems work in a co-ordinated fashion to respond to numerous threats from the environment. Synergy between their different mechanisms of action is essential for an intact, fully effective immune response [16]. For instance, different types of white blood cells are very important in the early immune response. The most important of these are the innate immune system cells monocytes and neutrophils. Both cell types are effective killers that secrete destructive substances including digestive enzymes, which can destroy the internalised material. Furthermore, pathogens can also be destroyed by antigen presenting B- and T-cells of the adaptive immune response. These cells include the functions of adaptation and memory, allowing the immune system to make specific responses and to remember individual types of infection. Thus, the immune system is a complex and strongly interconnected system, consisting of specialized tissues, organs (e.g. bone marrow, intestines), mobile and fixed immune cells, and a whole range of soluble proteins and molecules such as cytokines in order to protect the host from a range of dangerous pathogens such as bacteria and viruses. Despite this complexity, the immune system can be described as consisting of three main layers: first, epithelial barriers (e.g. airway mucus), second, cellular defenses and third, humoral responses such as antibody production [17–20].
A balanced supply of macronutrients and micronutrients is required for the development, maintenance and expression of the immune response. Conversely, poor nutrition and/or micronutrient deficiency compromise immune function and increase the risk for infection. Various micronutrients are essential for immunocompetence, particularly the vitamins A, C, D, selenium and zinc. This immunonutrition can improve the clinical course of surgical and critical ill patients and help the body to strengthen the immune system and have a greater protection and resistance against viral infections, especially in the elderly [6, 19, 20].
While there are many other immune-relevant micronutrients that are known to affect immune function, we have included only a selection in this review mainly based on the fact that these have been more intensively studied and their immunomodulating properties are widely accepted, especially in vulnerable groups (e.g. elderly). Immune-relevant micronutrients such as vitamin D, C, selenium or zinc may be used to support the immune system against viral respiratory tract infections and reduce the associated complications. Growing evidence from animal and human studies suggest that for some certain of these micronutrients increased intake above currently recommended levels may help optimize immune functions against respiratory tract infections [5–12, 15, 19, 20].
Malnutrition and micronutrient deficiency
The nutritional status can optimize the functioning of the immune system as a preventive measure by reducing both oxidative stress and inflammation that are involved in the pathogenesis of SARS-CoV-2. The evidence that deficiencies in micronutrients such as vitamin D, zinc and selenium may increase the risk for SARS-CoV-2 infection and COVID-19 disease mainly comes from epidemiological, experimental and observational studies, some case reports, and a few intervention studies. The first intervention studies indicate that high dose vitamin D supplementation reduces not only the mortality rate in critically ill patients with COVID-19 but also promotes viral clearance in asymptomatic and mildly symptomatic SARS-CoV-2 positive individuals. Further evidence emphasizing the role of vitamin D in reducing the risk of coronavirus disease include pathophysiological mechanisms such as: 1. The outbreak of COVID-19 occurred during winter months, at a time when 25(OH)D levels are low. 2. In the Southern Hemisphere the number of COVID-19 cases at the end of the summer are the lowest. 3. Vitamin D deficiency has been implicated as a pathogenic factor of acute respiratory distress syndrome (ARDS). The pooled odds ratio of ARDS decreases by 17% for every 1 nmol/L decrease in 25(OH)D (OR 0.83 (95% CI 0.69 to 0.98; p = 0.033)). 4. Case-fatality rates increase with age and co-morbidity, both are associated with vitamin D deficiency [21, 22].
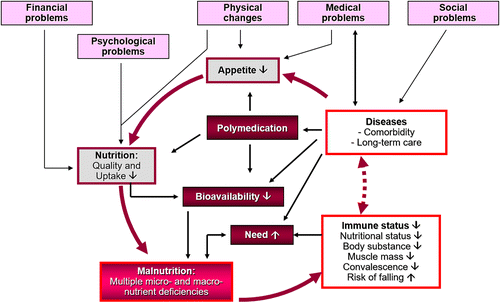
Malnutrition and poor nutritional status are widespread in older people and represent a major geriatric health problem with multifactorial etiology and severe consequences for quality of life. As macronutrients are the natural carriers of micronutrients, malnutrition is one of the main reasons that older patients have an inadequate micronutrient status. Age-related changes make elderly people more susceptible to malnutrition and infection. Although malnutrition can occur at any age, it is especially prevalent in people > 60 years of age. Poor nutritional status is well established as a negative prognostic indicator in the elderly population, and weight loss in individuals > 60 years of age approximately doubles the risk of dying [23, 24, 28, 29]. Furthermore, malnutrition is often characterised by protein catabolism and inflammation, which lead besides other predisposing factors to sarcopenia, the increased loss of muscle mass with aging [25, 26, 29]. In addition, malnutrition develops more quickly in older people than in younger persons and is more difficult to treat. Even a few days without an adequate supply of macronutrients (proteins, fats, carbohydrates) and micronutrients (vitamins, minerals, trace elements) can have serious effects on the immune status, nourishment, and composition of the body [26–30].
A systematic literature review of 54 studies from the period of January 1994 until December 2013 that used validated tools to screen community living adults (age: ≥ 65) for malnutrition susceptibility concluded that up to 83% are at risk for malnutrition [31]. In recent cross-sectional study the prevalence of malnutrition in elderly patients (age: 68.5 ± 8.8) with COVID-19 in Wuhan was evaluated using a Mini Nutritional Assessment (MNA) score. The patients were divided into a non-malnourished group (MNA ≥ 24), a group with increased risk of malnutrition (MNA 17–23.5) and a malnourished group (MNA < 17). Of the 182 included COVID-19 patients, 27.5% had a increased risk of malnutrition and 52.7% were malnourished [32]. The etiology of malnutrition and weight loss in the elderly has mainly been attributed to financial, medical, psychologic and social factors (see Figure 1). Physiological functions naturally decline with age, which can influence absorption and metabolism of macro- and micronutrients. Social and economic conditions can adversely affect dietary choices and eating patterns. However, at the same time, the need of the elderly for certain micronutrients (e.g. zinc, vitamin D) is higher than for younger adults. For example, the increase in the mean age is associated with an increasing number of multimorbid patients, who suffer from nutrition-associated diseases and usually depend on complex pharmacotherapy. About 40% of institutionalized patients take more than nine drugs on a daily basis. Under the most frequently prescribed drugs in the elderly are gastrointestinal agents, analgesics, antihypertensives, beta-blockers, antihyperlipidemic agents and diuretics. These drugs may cause a decreased food consumption and nutrient intake, absorption and utilisation, resulting in poor nutritional status. Given the ever-increasing number of drugs on the market and the frequency with which they are used, greater attention must be paid in daily medical and pharmaceutical practice focused in particular on the adverse effects of drug therapy on the micronutrient status [29, 33].
Nutritional problems must be recognised early and appropriate measures undertaken quickly. Therefore, the nutritional assessment and treatment should be a routine part of care for all, especially elderly persons, whether in the outpatient setting, acute care hospital, or long-term institutional care setting. As the risk for severe illness from COVID-19 increases with age, in particular vulnerable groups such as older adults should have their macronutrient and micronutrient status objectively determined with targeted replacement therapy if necessary. This paper makes no claim to completeness, but schematically presents the most important aspects in practice. The following selected micronutrients are clinically relevant for the prevention and treatment of respiratory tract infections of viral origin.
Immune-relevant micronutrients: A selection
Low serum levels of vitamin D, selenium and/or zinc appear to be an important risk factor for COVID-19 infection. Various observational studies observed an significant relationship between low serum concentration of 25(OH)D and susceptibility to morbidity and mortality caused by COVID-19. Also low serum levels of minerals such as selenium or zinc are associated with the increased mortality risk in patients with COVID-19. Among the immune-relevant micronutrients required to support a normal immune function, vitamin D, retinol, vitamin C and the trace elements selenium and zinc are of special importance [4–6, 15, 17, 18, 21, 29, 34, 35].
Vitamin D
Vitamin D insufficiency and deficiency is a major public health concern: Worldwide 40% are vitamin D deficient and 60% insufficient, i.e. serum level of 25-hydroxyvitamin D [25(OH)D < 20 and 20–29 ng/mL, respectively]. An estimated 1 billion people in the world, across all ethnicities and age groups, suffer from vitamin D deficiency. The pandemic of vitamin D deficiency/insufficiency can mainly be attributed to lifestyle and environmental factors that reduce exposure to sunlight, which is required for ultraviolet-B (UVB)-induced vitamin D production in the skin [36–41].
As expected in Europe, vitamin D deficiency and insufficiency are also widely prevalent during the winter months and affects mainly elderly people and migrants [41]. Several trials confirm the prevalence of vitamin D insufficiency in the European population and its associated potential health risk. For example, a prospective cohort study among elderly female patients (age 83.7 ± 6.1 yr) recruited from 95 nursing homes in Austria (n = 961) showed that the median 25(OH)D serum level was 17.5 nmol/L (=7 ng/mL). 95% of the institutionalized elderly patients had a 25(OH)D level < 50 nmol/L (<20 ng/mL). Lower serum levels of 25(OH)D were significantly associated with a higher all-cause mortality [42]. In relation to the coronavirus disease COVID-19 the calculated mortality rate from twelve European countries shows a significant inverse correlation with the 25(OH)D plasma level (P = 0.046) [15, 46]. In 2016, the ODIN study evaluated the 25(OH)D status of 55,844 Europeans. The results were alarming and a call to action for national and European healthcare policy makers: irrespective of age group, ethnic mix, and latitude of study populations 13% of the European individuals had a 25(OH)D status < 12 ng/mL, 40.4% had level of 25(OH)D < 20 ng/mL and 84% had a 25(OH)D status < 30 ng/mL. Compared with white populations (e.g. Norway, United Kingdom) the non-white population subgroups had a 3- to 71-fold higher yearly prevalence of vitamin D deficiency [43].
Black people absorb more UVB in the melanin of their skin than do white people and, therefore, require more sun exposure to produce the same amount of vitamin D. In the elderly a marked age-related decrease of cutaneous production of vitamin D can also be observed. A 70 year old has approximately only 25% of the 7-dehydrocholesterol that a young adult does and thus has a 75% reduced capacity to make vitamin D3 in the skin [44–46]. The observed rise of the COVID-19 pandemic in the elderly, African Americans and obese individuals suggests the possible impact of vitamin D status on host response and susceptibility to the infection as the elderly, black and obese individuals are known to have an elevated risk for vitamin D deficiency. Furthermore, the significant correlation between mortality from COVID-19 per million by country and latitude in countries south of 35 degrees North strongly supports the fact that vitamin D is a determining factor that influences severity of the coronavirus disease (P < 0.0001) [47].
Vitamin D status
25(OH)D is the vitamin D metabolite that is measured to assess a patient’s vitamin D status. Vitamin D deficiency is diagnosed when 25(OH)D < 20 ng/mL, vitamin D insufficiency is defined as 25(OH)D of 21–29 ng/mL, and 25(OH)D ≥ 30 ng/mL is considered sufficient, with 40–60 ng/mL being the preferred range. Vitamin D intoxication is only to be expected at levels of 25(OH)D > 150 ng/mL [36, 37, 39].
Based on different guidelines, the threshold for serum 25(OH)D has been set at 20–30 ng/mL for bone health. With respect to vitamin D’s non-skeletal effects, including the immuno-preventative effects, it has been suggested that a higher blood level of 25(OH)D of at least 30 ng/mL is required with the preferred range being 40–60 ng/mL. For improvement of the vitamin D status children and adults have to be supplemented. The Endocrine Society Practice Guidelines on Vitamin D recommends for infants, children 1 year and older and adults require 400–600 IUs, 600–1000 IUs and 1500–2000 IUs vitamin D daily. Obese people require 2–3 times more [36, 37, 39, 40].
It is unrealistic to believe that outdoor activities in the summertime will be able to raise serum 25(OH)D levels to such a significant extent that can be sustained throughout the winter. Typically serum levels of 25(OH)D increase by approximately 10–20 ng/mL by the end of the summer in white Europeans into the range of 35 ng/mL for those exposed to upwards of 300 hours of sunshine per month. Since the half-life for 25(OH)D is approximately 2–3 weeks serum levels decline below the desired 30 ng/mL within 1–2 months after October when sunlight can no longer produce any vitamin D in the skin for those living above 34° North latitude. Vitamin D3 supplementation with 2000 IU to 4000 IU vitamin D per day, respectively 40–60 IU vitamin D per kg bodyweight per day will increase serum 25(OH)D levels above 30 ng/mL [37–39].
The prohormone vitamin D
The fat-soluble vitamins D and A are different to other vitamins in that their bioactive metabolites, 1,25-dihydroxyvitamin D [1,25(OH)2D] and retinoic acid (RA) have hormone-like properties. Both of these steroid hormones are synthesized from their precursors by different cells and tissues in the body and exert their multiple effects on target cells by binding to nuclear receptors, such as the vitamin D receptor (VDR) or the retinoic acid receptors (RXR, RAR). After production in the skin or oral delivery the pro-hormone vitamin D affects important physiological functions in the body including modulation of the innate and adaptive immunity. In the liver vitamin D is metabolized to 25(OH)D by CYP2R1 and CYP27A1 with further metabolism in the kidney to the its biologically active metabolite 1,25-dihydroxyvitamin D [1,25(OH)2D] by CYP27B1. This metabolism can also occur in a variety of organs and tissues, including cells of the immune system [46].
As mentioned above, the secosteroid 1,25(OH)2D manifests its diverse biological effects (endocrine, autocrine, paracrine) by binding to the vitamin D receptor (VDR). The vitamin D receptor is expressed in most human tissues and has more than 1000 target genes. For example, vitamin D receptors have been found in over 35 tissues that are not involved in bone metabolism. These include endothelial cells, immune cells (e.g. monocytes, T-lymphocytes), islet cells of the pancreas, hematopoietic cells, cardiac and skeletal muscle cells, neurons and placental cells. It is estimated that VDR activation may regulate directly and/or indirectly a very large number of genes (up to 5% of the total human genome). The fact that the vitamin D receptor is expressed by many tissues results in the pronounced pleiotropic effect of vitamin D hormone [37, 38, 46]. 1,25(OH)2D influences cell metabolism via genomic and non-genomic metabolic processes. 1,25(OH)2D binds to the vitamin D receptor (VDR) and, after forming a heterodimer with the retinoid X receptor (RXR), translocates into the cell nucleus. Once there, it binds to the vitamin D responsive element (VDRE) in the DNA and regulates the transcription of numerous genes [50, 51, 64].
Vitamin D and Respiratory Tract Infections (RTIs)
Acute respiratory tract infections (e.g. influenza) are a major cause of global morbidity and mortality and are often responsible for ambulatory and emergency department visits. Airway epithelial cells are the first defensive barrier in the airway tract and play an important role in orchestrating neutrophil and macrophage recruitment to clear invading pathogens. It has been suggested that the seasonal variations in vitamin D levels could explain the increased prevalence of respiratory tract infections (RTIs) in the wintertime, when vitamin D synthesis is low. Observational and epidemiological studies, supported by the results of several interventional studies have demonstrated a strong association between 25(OH)D serum levels and the incidence of respiratory tract infections.
In a systematic review and meta-analysis of 11 randomized controlled trials with 5660 patients vitamin D showed a significant protective effect against RTI (OR, 0.64; 95% CI, 0.49 to 0.84). The protective effect was larger in studies using once-daily dosing compared with bolus doses (OR = 0.51 vs OR = 0.86, P = 0.01) [52]. In another systematic review and meta-analysis (n = 11321, age: 0–95 years) of 25 randomised controlled trials Vitamin D supplementation reduced significant the risk of acute respiratory tract infection (adjusted OR = 0.88, 95% CI 0.81 to 0.96; P < 0.001). In subgroup analysis, protective effects were also stronger in those receiving daily vitamin D without additional bolus doses [53]. Furthermore, animal studies have shown that the response to a variety of vaccine preparations could be increased by vitamin D. For example, in mice immunized with an attenuated influenza virus vaccine a Vitamin D and Vitamin A deficiency reduced antibody response in the respiratory tract to a greater extent than a deficiency for one of these vitamins. Although supplementation with vitamin A had a greater corrective effect than vitamin D for the restitution of seroprotection (e.g. IgG, IgA response), the best results were obtained with the two vitamins combined [54, 55].
Vitamin D and COVID-19
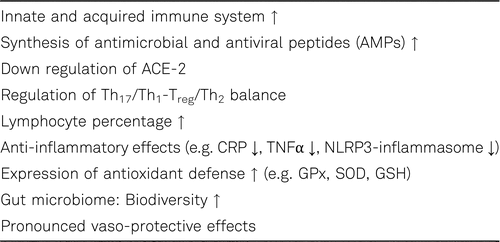
Vitamin D insufficiency and deficiency are common in COVID-19 patients and correlate with increased risk for SARS-CoV-2 infection as well as the progression and severity of COVID-19 [49, 56]. In a retrospective observational study that included adult COVID-19 patients from Turkey (n = 149) vitamin D insufficiency [25(OH)D < 30 ng/mL] was present in 93.1% of the patients with severe-critical COVID-19 [57]. 1,25(OH)2D exerts its effects on immune system by modulating of both the adaptive and innate immune systems by the regulation of cell signalling pathways (see Table 1). For instance, 1,25(OH)2D increases the synthesis of antimicrobial peptides (AMPs), with antibacterial, antifungal and antiviral activities. The production of AMPs such as denfensins and cathelicidin (e.g. LL-37) haves antiviral effects and lowers the infectivity of respiratory viruses such as influenza. [58].
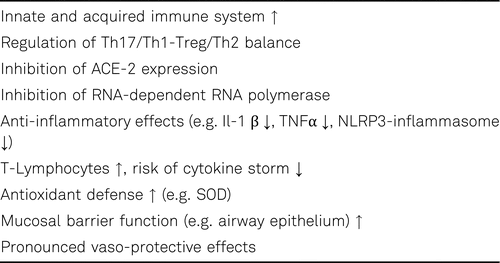
Inflammation and oxidative stress play an important role in SARS-CoV-2 virus replication and COVID-19 progression. Reactive oxygen species (ROS) are directly linked through activation of NLRP3 inflammasome to inflammation. Vitamin D deficiency enhances oxidative stress, impairs mitochondrial function and increases systemic inflammation. As one of the key controllers vitamin D modulates systemic inflammation, mitochondrial respiratory function and oxidative stress. 1,25(OH)2D mediates many of its anti-microbial, anti-oxidant and anti-inflammatory effects including inhibition of IL-1β, IL-6, IL-17, TNFα and INFγ production through the vitamin D receptor. Furthermore 1,25(OH)2D inhibit the mitogen-activated protein kinase (MAPK) and NF-kB signalling [59, 60]. In addition, vitamin D negatively regulates the NLRP3 inflammasome via VDR signaling to effectively inhibit IL-1β secretion [61, 62].
Type II pneumocytes in the lungs, goblet secretory cells in the nasal passages, and the absorptive enterocytes in the intestines are potential targets of the coronavirus. Spike proteins of the virus facilitate viral entry into these cells through binding with the angiotensin converting enzyme 2 (ACE-2) on the surface. ACE-2, a regulator of the renin-angiotensin system is distributed in many tissues in the body including lung, kidney, gastrointestinal (GI) tract, and cardiovascular system that could explain multi-organ failure in susceptible patients to COVID-19. 1,25(OH)2D also acts as a negative endocrine regulator of renin-angiotensin system and down-regulates ACE-2 [48, 63, 64]. Several members of the gut microbiota help also protect against respiratory tract infections. 1,25(OH)2D plays a key role in controlling and regulating genes responsible for preserving the integrity of the epithelial barrier including the gastrointestinal tract, in addition to immunoregulatory and inflammatory responses. Vitamins D and Vitamin A are of particular importance for the barrier function of mucous membranes in the respiratory, intestinal and urogenital tract. Furthermore, 1,25(OH)2D induces the expression of the antioxidant defense system including catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD), increases the levels of glutathione (GSH), and thereby contributing to reduce oxidative stress and cellular oxidation [62]. Vitamin D modulates the gut microbiota, which can decrease gut permeability and inflammatory status. The supplementation of vitamin D increases the biodiversity of the gut microbiome (e.g. bacteriodes), which means their resistance against stressors and intestinal inflammation is enhanced [65]. The concentration of spermidine, a novel autophagy inducer, declines with age in cells and organs resulting in a decrease of autophagy. Interestingly, 1,25(OH)2D can induce autophagy similar to spermidine by increasing Beclin-1 expression and inhibiting mammalian target of rapamycin 1 (mTORC1) complex activation, similar to spermidine [67, 68].
Meanwhile more than 40 intervention studies have started to test the effect of vitamin D on the progress of the COVID-19 disease. In a recent trial that used a retrospective, observational analysis of deidentified tests performed at a National American clinical laboratory to determine if circulating 25(OH)D levels are associated with severe acute respiratory disease coronavirus 2 (SARS-CoV-2) positivity rates. A total of 191,779 patients from all 50 states with SARS-CoV-2 were analyzed (median age, 54 years) and the results performed mid-March through mid-June, 2020 and matching 25(OH)D results from the preceding 12 months were included (see Figure 2) [56]. The results demonstrate an inverse relationship between circulating 25(OH)D levels and infection with SARS-CoV-2. SARS-CoV-2 positivity was strongly and inversely associated with circulating 25(OH)D levels, a relationship that persists across latitudes, races/ethnicities, both sexes, and age ranges.
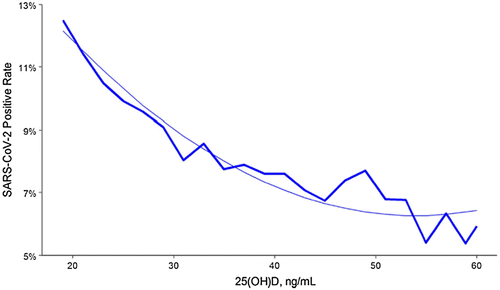
For the entire population those who had a circulating level of 25(OH)D < 20 ng/mL had a 54% higher positivity rate compared to those who had a blood level of 30–34 ng/mL. The risk for SARS-CoV-2 infection continued to decline until the serum levels reached 55 ng/mL. This finding is not surprising, given the established inverse relationship between risk of respiratory viral pathogens, including influenza, and 25(OH)D levels. Vitamin D supplementation may reduce acute respiratory infections, especially in people with vitamin D insufficiency or deficiency. A previous study found that each 4 ng/mL increase in circulating 25(OH)D levels was associated with a 7% decreased risk of seasonal infection, a decrement of approximately 1.75% per ng/mL. This is remarkably similar to the 1.6% lower risk of SARS-CoV-2 positivity per ng/mL found in this adjusted multivariable model [56].
Another recent retrospective study from Indonesia with 780 older male patients of COVID-19 showed that the mortality rate dropped to almost to 0% when the serum 25(OH)D concentrations were higher than 34 ng/mL [49].
In a cross-sectional study from September 2020 the data from Sinha Hospital COVID-19 Registry in Teheran analyzed the data of 235 patients infected with COVID-19 (mean age: 58,7 years). Based on CDC criteria, among this Iranian patients, 74% had severe COVID-19 infection and only 32,8% were vitamin D sufficient [25(OH)D ≥ 30 ng/mL]. After adjusting for confounding factors, there was a significant association between vitamin D sufficiency and reduction in clinical severity, inpatient mortality, serum levels of C-reactive protein (CRP) and an increase in lymphocyte percentage [48].
In a randomized, placebo-controlled vitamin D3 intervention study (SHADE study) with asymptomatic and mildly symptomatic SARS-CoV-2 positive individuals (n = 30), it was found that high dose vitamin D supplementation (60,000 IU daily for 7 days) in order to reach serum levels of 25(OH)D > 50 ng/ml helps to achieve SARS-CoV-2 RNA negativity in greater proportion of asymptomatic vitamin D-deficient individuals with SARS-CoV-2 infection along with a significant decrease in inflammatory marker. Thus, high-dose Vitamin D3 supplementation led to SARS-CoV-2 RNA negative in additional 41.7% participants (p < 0.001) and was useful to increase viral SARS-CoV-2 RNA clearance [69].
In a recent prospective observational study 25(OH)D serum levels were significantly reduced in critically ill COVID-19 patients (14.35 ± 5.79, P = 0.0001), whereas the intensity of the inflammatory response was increased. When the case fatality rates were compared on the basis of vitamin D deficiency, the fatality rate was 21% (19 patients died in 90 patients) among vitamin D deficient and 3.1% (2 patient died in 64) among patients with normal vitamin D level. The results of this study show an increased morbidity and mortality in COVID-19 patients with vitamin D deficiency [70].
A quasi experimental study with 66 patients (mean age: 87.7 ± 9.0 years) showed a clinically relevant protective effect of a bolus of vitamin D3 (e.g. 80,000 IU), that has been supplemented just before or during coronavirus disease 2019 in order to reduce COVID-19 induced mortality. The hazard ratio (HR) for mortality according to vitamin D3 supplementation was 0.21 [95 % confidence interval (95%CI): 0.07; 0.63] P = 0.005]. Furthermore, the residents who had not recently received vitamin D3 supplements had a shorter survival time (P = 0.002) and the bolus vitamin D3 supplementation during or just before COVID-19 was inversely associated with the Ordinal Scale for Clinical Improvement (OSCI) score for COVID-19 in the acute phase [71]. The results of further intervention studies with vitamin D supplementation are urgently expected [72].
The multiple anti-inflammatory and immunomodulatory effects of Vitamin D could explain its protective role against immune hyper reaction and cytokine storm in patients with severe COVID-19 [56, 73, 74]. Vitamin D reduces serum CRP and increases lymphocytes percentage. Vitamin D supplementation may help in treatment of COVID-19 by preventing the cytokine storm and subsequent ARDS which is commonly the cause of mortality [48, 56, 62–67, 73, 74].
Although it would be helpful to monitor serum 25(OH)D levels in all patients in particular the elderly and hospitalized patients with COVID-19 to identify those who are Vitamin D deficient there is enough evidence to suggest that most are. Since the assay for 25(OH)D is not always available and can be expensive there is no reason not to be giving on patients with COVID-19 vitamin D supplementation along the guidelines recommended by the Endocrine Society Practice Guidelines on Vitamin D. The results of the first intervention studies with vitamin D supplementation will help provide guidance for the use of the sunshine vitamin during this pandemic. Treatment with vitamin D appears promising because of its role as anti-inflammatory, antioxidant, immunomodulatory agent, and regulator of vascular homeostasis. Vitamin D3 supplementation may represent an effective, accessible and well-tolerated adjuvant treatment for COVID-19. Keeping the current COVID-19 pandemic in view we recommend therefore the administration of vitamin D supplements to vulnerable groups at high risk for COVID-19 such as the elderly [37, 48, 56, 62–68, 73, 74].
Recommendation for clinical practice
Prevention
To help reduce risk for viral infections of the respiratory tract, elderly people, children, adolescents, and adults should supplement vitamin D as recommended by the Endocrine Society. Vitamin D3 supplementation with 2000 to 4000 IU per day, respectively 40 to 60 IU vitamin D per kg bodyweight per day will increase serum 25(OH)D levels above 30 ng/mL.
Supportive treatment: Hospital admission, severe course of COVID-19
Based on the information to date where blood levels of 25(OH)D of up to 60 ng/ML can reduce risk of infection by as much as 54.5% it is reasonable to give all patients presenting with COVID-19 an initial bolus dose of between 50,000 and up to 200,000 IUs of vitamin D. To achieve a blood level in the preferred range of 40–60 ng/ML requires ingestion of between 4000–6000 IUs daily. It is reasonable to give patients 10,000 IUs daily or the equivalent of weekly 50,000–60,000 IUs during the hospitalization. To maintain a blood level in the preferred range the patients should be encouraged to continue taking this amount of vitamin D after leaving the hospital [37, 38, 56, 76].
Vitamin A (Retinol)
According to World Health Organization (WHO) vitamin A deficiency (VAD) is a major public health problem, especially in children and pregnant women, in more than 50% of all countries (esp. Africa, South-East Asia). In children vitamin A deficiency is the leading cause of preventable blindness, increases the risk of disease and death from severe infections [75]. But the problem of micronutrient deficiencies is not only limited to low- and middle-income countries. In affluent countries, such as Germany, the Netherlands, the United Kingdom (UK) and the USA, the evaluation of National Surveys underlines that modern lifestyle and environmental factors may also promote suboptimal vitamin A intakes. For example, over 75% of all adults in the USA do not meet the recommendations for vitamin A (0.9 mg = 3.000 IU vitamin A daily). In the Netherlands and the UK over 50–75% does not receive daily recommended intakes, and in Germany up to 25% of the population does not receive the daily recommended intakes for vitamin A (1 mg = 3.333 IU vitamin A daily) [76]. It can be assumed that the suboptimal intake of vitamin A and/or vitamin A deficiency is also prevalent among the elderly [77–79]. In the elderly vitamin A deficiency is associated with defective immune response to infections with pathogens and correlates with an increased risk for cognitive decline [77–81].
Besides the reduced dietary vitamin A intake a disturbed effectiveness to convert provitamin A (beta carotene) into retinol can help to explain the high prevalence of subclinical vitamin A deficiency. Previous nutritional surveys used a conversion factor of 6:1 (6 mg beta carotene = 1 mg retinol) to calculate the vitamin A activity from the beta carotene intake. But a frequent genetic polymorphism of the enzyme β-carotene 15,15’-monoxygenase (BCMO) influences the provitamin A conversion efficiency and is known to affect some 45% of the Caucasian race [81, 82]. Those affected are hardly able to convert beta carotene into retinol, and must therefore cover their nutritional need of vitamin A instead of plants from animal foods. Recent studies have shown that a more realistic conversion factor is 28:1 (28 mg beta carotene = 1 mg retinol) [83, 84]. As a consequence, single nucleotide polymorphisms can strongly influence the effectiveness of using plant-based provitamin A carotenoids to increase vitamin A status and should always be taken into account in high risk groups for vitamin A deficiency.
Vitamin A status
In contrast to the vitamin D status that can be assessed easily by 25(OH)D in serum, the assessment of vitamin A status is more complicated. Vitamin A is mainly stored in the liver, kidney and lung tissue in high quantities ranging between 10–1000 nmol/g, of which the lung is the second major retinoid storage organ. Remarkably, the bioactive form of vitamin A, retinoic acid (RA) can help to support human lung regeneration [85, 86]. As vitamin A is primarily stored in hepatic stellate cells, liver biopsies have often been done under surgical conditions to assess vitamin A status. However, for obvious reasons, a liver biopsy (gold standard) cannot be widely used. Therefore, serum retinol concentration is the common method used to evaluate vitamin A deficiency, but it is homeostatically controlled until liver reserves become dangerously low. Nearly all retinol, the circulating form of vitamin A in serum, is bound to retinol-binding protein (RBP), so RBP concentrations in blood can also be used as an indicator of vitamin A status. The RBP in serum is a common biomarker used for assessment of vitamin A status. A serum RBP concentration lower than 14.7 μg/mL (0.70 μmol/L) indicates a vitamin A deficiency [87]. But RBP concentrations in blood can be temporarily reduced by acute infection and inflammation. Therefore, C-reactive protein (CRP) or similar markers of inflammation should also be measured, to correctly interpret vitamin A status. The RBP:transthyretin ratio may also help to determine if serum retinol concentrations are depressed by infection [87–90].
Vitamin A: Selected functions
Vitamin A is essential for a variety of functions such as vision, spermatogenesis, female reproduction, embryonic development, normal growth and development of infants, immunity, and differentiation, reproduction and maintenance of epithelial cells. In view of the immunmodulatory effects the retinoid X receptor (RXR) often forms a heterodimer with the vitamin D receptor (VDR), so that the influence of numerous cell functions of the immune system (esp. adaptive immunity) by vitamin A and D are closely related [64, 91–95].
Vitamin A exists in three forms: retinol, retinal, and retinoic acid (RA), the latter being the most metabolically active. Retinol is the main transport form of Vitamin A, retinal is the form involved in the visual cycle (rhodopsin cycle), whereas RA is involved in regulation, differentiation, maturation and function of cells (e.g. immune cells). The liver is the major storage for vitamin A. For storage retinol is transported from hepatocytes to the stellate cells and converted to retinyl esters (e.g. retinyl palmitate). Transformation of retinol into bioactive RA involves a two-step oxidative reaction. The first step of RA synthesis is controlled by enzymes of the alcohol dehydrogenases (ADH) or short-chain dehydrogenase/reductase (SDR) families that catalyze the reversible oxidation from retinol to retinal (retinaldehyde). The second step of RA synthesis is the irreversible oxidation from retinal to RA by enzymes of aldehyde dehydrogenase (RALDH) family. RA exists in two forms: 9-cis-RA and all-trans-RA (ATRA). ATRA and 9-cis-RA are the potent regulators of gene expression and play an essential role in the modulation of cell proliferation and differentiation [96, 97].
Vitamin A and infectious disease
Vitamin A is an important regulator of immune protection. The fat-soluble vitamin A is commonly known as “the anti-infective vitamin”, due to its importance for normal functioning of the innate and adaptive immunity. The impact of vitamin A deficiency on immunity has been extensively studied and proven the indispensable requirement of this vitamin to maintain host defense to viral, bacterial, and protozoal diseases [98–100]. For instance, a longitudinal cohort study of tuberculosis revealed that Vitamin A deficiency is associated in a dose dependent way to the occurrence of tuberculosis [101]. Additionally, in patients with tuberculosis often a combined deficiency of vitamin A and D can be found [102].
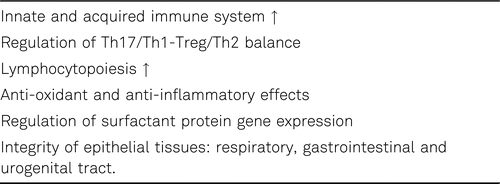
Vitamin A deficiency is associated with multiple alterations in immune response, including pathological alterations in mucosal surfaces, impaired antibody response, changes in lymphocyte populations, and altered B- and T-cell function. Epithelial tissues cover most of the external and internal surfaces of the organs. These tissues serve as first line of defence against pathogen invasion (e.g. viruses). Vitamin A plays a crucial role for the morphological formation and integrity of epithelial cells. Therefore it is an integral part of the mucus layer, in the respiratory, the gastrointestinal and the urogenital tract. Vitamin A promotes mucin secretion and improves the antigen non-specific immunity function in these tissues [103]. Gut-associated dendritic cells (DCs) can synthesize retinoic acid (RA). RA induces the expression of gut-homing receptors (e.g. α4β7-integrin, CCR9) followed by lymphocytes activation [104].
Altogether, vitamin A improves the defense of the mucous membranes, reduces the intestinal permeability and increases the integrity of alveolar and intestinal mucusa. Furthermore, vitamin A can help to restore lung surfactant. In vitamin A deficiency the resistance of epithelial tissues to foreign pathogens decreases, and it is no longer able to exert its mechanical barrier function, thus reducing innate immune function and promoting respiratory tract infections. Consequently, marginal vitamin A status clearly impairs the integrity of epithelial tissues [105, 106].
The vitamin A metabolite retinoic acid (RA) has regulatory and supporting roles in both adaptive and innate immune response. For example, vitamin A plays a crucial part in the regulation of the maturation, differentiation and function of cells of the innate immune system. It is known, that innate immune cells are comprised of macrophages and neutrophils, which initiate immediate responses to pathogen invasion through phagocytosis and activation of natural killer T cells which perform immunoregulatory functions through cytotoxic activity. The immune-supporting roles of vitamin A include the promotion of antibody production, cytokine expression, lymphocytopoiesis and the enhanced functions of natural killer cells, monocytes/ macrophages, neutrophils, B and T cells. Similar to the hormone 1,25(OH)2D, retinoic acid (RA) also is required for adaptive immunity and plays a role in the development of both T-helper (Th) cells and B-cells. RA modulates the Th1:Th2-cell balance, induces Th2-cell response and inhibits Th17-cell differentiation. Therefore, a vitamin A deficiency is associated with a decreased Th2-cell response. Conversely, the supplementation of vitamin A blocks the production of Th1-cell cytokines. The complex immunmodulatory effects of retinoic acid have been extensively reviewed in [107, 108].
People with vitamin A deficiency are prone to increased risk and severity to viral infections, including the influenza virus, human respiratory syncytial virus (RSV) and measles virus. Populations at highest risk for severe RSV infection include the elderly (65 years and older), adults with chronic heart or lung disease, and adults with weakened immune systems. The alterations in mucosal regeneration and immune response presumably account for the increased mortality and morbidity in vitamin A deficiency due to viral infections [97–102]. In a meta-analysis, vitamin A supplementation was associated with a clinically meaningful reduction in morbidity and mortality in children under five years. For example, vitamin A supplementation reduced the incidence of diarrhoea by 15% (RR 0.85, 95% CI 0.82 to 0.87), the incidence of measles by 50% (RR 0.50, 95% CI 0.37 to 0.67) and risk for all-cause mortality by 12% (RR 0.88; 95% CI 0.83 to 0.93) [103, 104]. As vitamin A is crucial for normal differentiation of epithelial tissues and maintenance and functioning of the innate and adaptive immune response, the supplementation of vitamin A may be helpful against viral respiratory tract infections, such as COVID-19 (see Table 2) [100, 101, 106–110].
Vitamin A and COVID-19
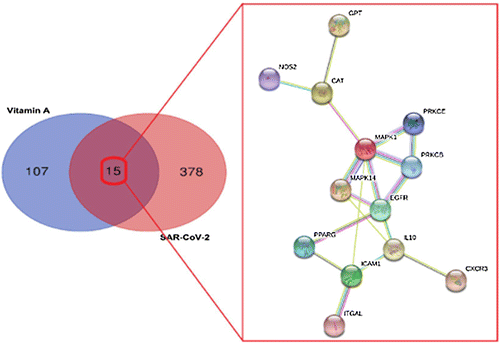
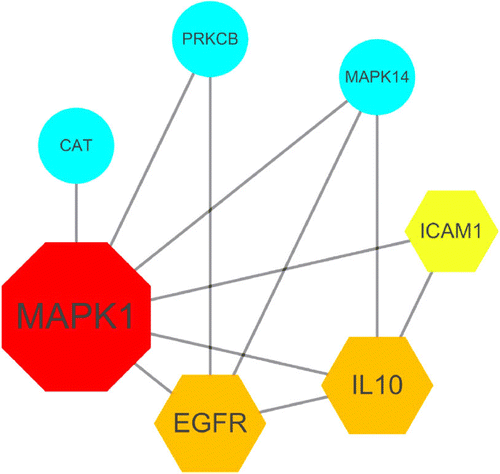
Oxidative stress and inflammation are key players in the pathogenesis and progression of Coronavirus disease 2019 (COVID-19) [111–113]. The burden with ROS and pro-inflammatory conditions can negatively affect vitamin A supply through increased consumption, reduced intestinal absorption, increased urinary excretion and sequestration in the liver [114–116]. In a recent study, computation assays and bioinformatics analysis using a network pharmacology method were conducted to uncover the therapeutic targets and mechanisms of vitamin A for treating COVID-19. In this process candidate targets, pharmacological functions, and therapeutic pathways of vitamin A against SARS-CoV-2 were indentified (see Figures 3 and 4). The bioinformatics results indicate that the mechanisms of action of vitamin A against SARS-CoV-2 include inhibition of pro-inflammatory processes, immunmodulatory and anti-oxidant effects. Above that seven core targets of vitamin A against COVID-19, including catalase activity (CAT), epidermal growth factor receptor (EGFR), intercellular adhesion molecule 1 (ICAM1), IL10, mitogen-activated protein kinase 1 (MAPK1), mitogen-activated protein kinase 14 (MAPK14) and protein kinase C beta type PRKCB were identified. This bioinformatics-based report reveals the anti-SARS-CoV-2 mechanisms of vitamin A suggest that this vitamin may act as a potent treatment option for COVID-19 [117].
Recommendation
Prevention
In order to prevent a viral infection of the respiratory tract we recommend elderly people, adults and adolescents to supplement 2,000–4,000 IU vitamin A (retinol) per day.
Supportive treatment: Hospital admission, severe course of COVID-19
a) Initially (day 1, bolus): Based on the information to date it is reasonable to give all patients presenting with COVID-19 an initial bolus dose of between 50,000 and up to 200,000 IUs of vitamin A (retinol).
b) Followed by: 10,000 IU vitamin A daily for 1 month; then: 5,000 IU vitamin A per day administered orally.
Vitamin C
Epidemiological studies carried out in Europe and North America have indicated that hypovitaminosis C (<23–28 μmol/L) or dietary inadequacy (<50 μmol/L) is common in Western populations. According to several National Diet and Nutritional Surveys in countries such as Germany, the Netherlands, the UK or the USA where food availability and supply should be expected to be sufficient, the dietary intakes for vitamin C is often suboptimal. For example, over 50–75% of all adults in the USA do not meet the daily recommendations for vitamin C (90 mg vitamin C daily) and in the Netherlands and Germany over 50–75% of the adults do not receive the daily recommended intakes for Vitamin C (70 mg resp. 100 mg vitamin C daily) [76, 118, 119]. Among the reasons why dietary recommendations for vitamin C are often not met are: Economic reasons (poor socioeconomic status, limited access to micronutrient dense food), poor dietary habits, lifestyle limiting intakes or increasing vitamin C requirements (e.g., drugs, alcohol abuse), exposure to pollutants and smoke (both passive and active) and various diseases (e.g. infectious diseases, diabetes). In elderly hospitalized patients (age > 65 years) the prevalence of vitamin C deficiency can reach up to 80%. Epidemiologic evidence indicates that a greater intake of vitamin C decreases the risk of cardiovascular disease and morbidity from stroke [119, 120]. Several studies have indicated that compared with healthy individuals critically ill patients have very low circulating vitamin C levels. Pharmacokinetic studies in critically ill patients reveal that the parenteral administration of vitamin C (e.g. 2–10 g Vitamin C per infusion) is needed to keep their plasma level under such conditions in a normal range [121].
Vitamin C and diet
A diet that supplies 200 mg vitamin C daily is adequate to saturating immune cell and plasma concentrations (≥70 μmol/L) in healthy individuals and should cover general requirements for the reduction of chronic disease risk. T lymphocytes and other immunocompetent cells are able to accumulate vitamin C [123–128, 131]. The vitamin C concentration in these cells is 10–100 times higher than in the blood. At this level, not only the immunocompetent cells (e.g. neutrophils, lymphocytes) are saturated with vitamin C but also the risks of cardiovascular disease or cancer and the all-cause mortality is reduced [123–128, 131]. In order to attain such blood levels, healthy people have to consume some five portions of fresh vegetables and fruit (e.g. gooseberries, sweet peppers, kiwi, broccoli) each day or take a supplement of 200 mg vitamin C (e.g. by drinking ¼ teaspoon of vitamin C powder dissolved in freshly pressed orange juice).
Vitamin C and immunity
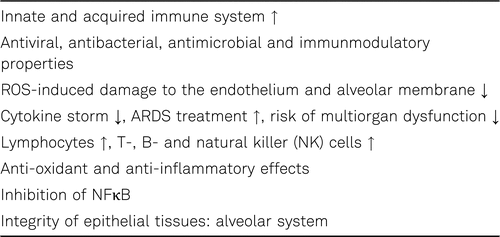
Vitamin C (ascorbic acid) is not only an important antioxidant vitamin but is also crucial for both innate and adaptive immunity. Several aspects of immunity, including the supporting epithelial barrier function (e.g. alveolar membrane), endothelial protection, phagocytosis, white blood cell migration to sites of infection, microbial killing, and antibody production are influenced by vitamin C. At the humoral level, vitamin C enhances antibody production (IgA, IgM) and complement component C3 in the blood. Vitamin C also increases interferon production and defence mechanisms against viral infections. Lymphocyte proliferation and maturation are stimulated by vitamin C [131, 134].
In addition, vitamin C increases phagocytosis and chemotaxis in neutrophils, eosinophils, and monocytes [118, 125, 126, 134]. Vitamin C deficiency increases the risk and severity of viral infections (e.g. influenza), the risk of oxidative damage to membranes (e.g. alveolar system) and endothelial dysfunction, and the pro-inflammatory cytokine load (e.g. TNFα) [118, 127–131, 134]. Intravenous vitamin C may positively impact the extent of multiple organ failure and enhances the expression of tight junctions, increases epithelial barrier integrity which helps to restore pulmonary function [133, 134]. Thus, vitamin C can favourably expand the treatment options in patients with viral pneumonia and ARDS in severe SARS-CoV-2 infection by decreasing oxidative stress and inflammation, increasing the immune defence and endothelial integrity, reducing tissue and organ injury, and improving the overall outcome of the disease [134].
Vitamin C and COVID-19
A growing body of evidence suggests that a cytokine storm, which is a potentially fatal immune reaction triggered by a variety of factors (e.g. infection) is associated with COVID-19 progression and severity, and is often the cause for death [135]. Cytokine storms are associated with severe inflammation and elevated levels of pro-inflammatory cytokines. Thus, cytokine storms play a main mechanism of highly pathogenic human coronavirus infected pneumonia, such as severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) [135, 136, 138]. Moreover, clinical characteristics of COVID-19 indicate that cytokine storms are positively correlated with the severity of the disease [137–139]. SARS-CoV-2 can infect monocytes and macrophages through ACE2-dependent and ACE2-independent pathways. Thereby the anti-viral response of monocytes and macrophages is suppressed. Additionally the adapative immune response is impaired by the infection with SARS-CoV-2, since monocytes and dendritic cells (DCs) act as antigen presenting cells (APCs). For example, elevated circulating quantities of IL-1β, IL-10, IL-17, TNF-α, G-CSF, GMCSF, CXCL8, CXCL10 and IFN-γ have been detected in the COVID-19 patients, especially in those needing intensive care unit (ICU) facilities. Several of these chemokines and cytokines released through monocytes and macrophages can escalate the pathogenesis of COVID-19 [139].
As mentioned above vitamin C has complex immunmodulatory, antiviral, antibacterial, antioxidativ, and anti-inflammatory properties, especially in high concentrations (see Table 3) [140]. Vitamin C can inhibit the activation of redox-sensitive nuclear factor kappa-B (NFκB), which is a key pro-inflammatory transcription factor to produce inflammatory mediators (e.g. cytokines, chemokines) [127, 140]. Vitamin C inhibits granulocyte macrophage-colony-stimulating factor (GM-CSF) and mitigates tumor necrosis factor-alpha (TNF-α) in severe community acquired pneumonia [141, 142]. In addition, ascorbic acid regulates the function and proliferation of T cells, B cells and natural killer (NK) cells (see Table 3) [143].
The intravenous administration of vitamin C achieves higher blood levels (>1000 μmol/L) and has gained its place in complementary medicine as a supportive treatment for respiratory tract infections (e.g. 7.5 g vitamin C in 100 mL 0.9% NaCl, 2–4 times/week). Further, intensive care medicine has shown that vitamin C infusions improve the locomotor properties of immune cells (e.g. neutrophils in sepsis) [148, 149, 151, 152]. Recent meta-analysis indicated that vitamin C may shorten the common cold, the length of ICU stay and the duration of mechanical ventilation in critically ill patients [144–148, 151, 152]. Interestingly, the Shanghai government now officially recommends high-dose vitamin C therapy (100–200 mg/kg body weight/day, i.v.) in their guidelines for the treatment of Covid-19 [149, 150, 152, 157].
Vitamin C can help to suppress cytokine storms, one of the main mechanisms in the deterioration of patients with COVID-19. Therefore, the application of intravenous vitamin C at an early stage of ARDS in patients with COVID-19 may help the management of cytokine storms, improve the host’s immunity and help in better outcomes [134, 152–154, 156, 157]. The results of the first randomised interventional studies with vitamin C infusions (e.g. 12 g vitamin C intravenously twice daily for 7 days) are eagerly awaited [133, 134, 150, 155–157]. As vitamin C offers a safe and inexpensive treatment option, and has several potential positive effects on COVID-19, clinicians should be encouraged to use vitamin C infusions in ICU along with the other treatments (e.g. corticosteroids), especially until we have a vaccines.
Recommendation
Prevention
1. Oral supplementation: 1000–3000 mg vitamin C (e.g. plus quercetin, in divided doses throughout the day).
Supportive treatment: hospital admission, severe course of COVID-19
2. a) Initially (days 1–10): 15–30 g vitamin C per day as an intravenous infusion (e.g. in 100–200 mL 0.9% NaCl as a short infusion) with prior exclusion of a glucose-6-phosphate dehydrogenase deficiency and any contraindications to vitamin C (e.g. haemochromatosis, renal failure).
b) Then: 2–4 infusions with 7.5–15 g vitamin C (e.g. in 100–200 mL 0.9% NaCl as a short infusion) per week.
Selenium
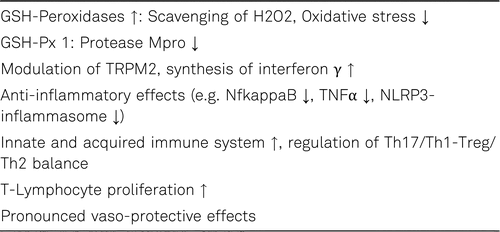
Selenium is an essential trace element that has various important aspects for human health, including antioxidant, anti-inflammatory, immunomodulatory, anti-carcinogenic and antiviral properties. The health promoting effects of selenium and its compounds are due to its unique mechanism of incorporation as the 21st proteinogenic amino acid selenocysteine in selenoproteins encoded by 25 separate human genes (human selenoproteome) with roles in cell protection from oxidative stress, metabolic control, and the inflammatory response. Amongst the 25 selenoprotein genes identified to date several have important functions in antioxidant defense, redox homeostasis and cell signalling such as the glutathione peroxidases (GSH-Px 1-4, 6), that reduce hydrogen and lipid peroxides, iodothyronine deiodinases (DIO), that activates thyroid hormones, thioredoxin reductases (Trx-R 1-3), that are essentials in the homeostasis of thiol systems, and selenoprotein-P (SELENOP), which is the main carrier of selenium to target organs. Selenium-dependent GSH-Px and Trx-R are very important for optimal function of immune cells by controlling oxidative stress and redox regulation [158, 159].
Selenium has a narrow therapeutic window. Although normally safe, the supplementation may have adverse effects in individuals that have already an adequate dietary selenium supply. In rare cases selenium can cause hair loss, fatigue, neurological disturbance (e.g. restlessness), gastrointestinal side effects (e.g. vomiting, diarrhoea) and has been associated in persons with high baseline selenium levels with an increased risk of type 2 diabetes. However, suboptimal intake of selenium is widespread in many parts of the world (e.g. Europe). In Europe for example, consuming a balanced diet will barely deliver more than 45 μg selenium per day to an adult. Correspondingly low are the average serum levels in the populations of Greek, the Netherlands and Germany 55 μg/L, 65 μg/L, and 75 μg/L respectively [160]. In 1985, Finland started a population-wide selenium supplementation effort and raised the average plasma selenium concentration from around 70 μg/L to levels of around 111 μg/L. Worldwide, it is estimated that about one billion people suffer from selenium deficiency (<100 μg/L). The optimal range of selenium status according to Rayman has been defined by a selenium serum level between 130 to 150 μg/L [161]. Until the year 2099 the selenium-poor soils (e.g. Europe) will lose even further selenium and other minerals as a result of climate change, particularly in agricultural areas [162–164]. The low selenium intake causes insufficient expression of selenoproteins (e.g. selenoprotein P), low selenium levels in the circulation and tissues, as well as an increased risk for several chronic diseases. Selenium deficiency has been recognized as a contributing factor to different pathophysiological conditions, such as cancers, cardiovascular, thyroid, neurodegenerative and/or infectious diseases, inflammation, and immunodeficiency [158, 163–165].
Selenium and viral infection
Viral infections are accompanied by alteration of intracellular redox state and induction of ROS-generating enzymes, such as the NADPH oxidases/dual oxidases (NOX/DUOX) and xanthine oxidase (XO), that promote the production of H2O2 and disturb subsequently antioxidant defenses. The main H2O2 scavenging enzymes are the glutathione peroxidases (e.g. GSH-Px 1) and the thioredoxin reductases (Trx-Rs). For instance, among the viruses that increase oxidative stress are influenza viruses, respiratory syncytial virus (RSV) and human immunodeficiency virus (HIV). Selenium deficiency is an established risk factor for viral infections. As is well known host selenium deficiency might increase the virulence of RNA viruses such as coxsackievirus B3 and influenza A [166]. Selenium levels may also affect B-cell-dependent antibody production. A selenium deficiency causes a weakening of the immune response and reduces the body’s chances for dealing with viral infections. Furthermore, a selenium deficiency may promote viral mutations (e.g. influenza). Under conditions of selenium deficiency viruses can mutate, replicate and spread more quickly in the body. As a consequence the course of a viral infection is more serious [164–170].
Selenium and COVID-19
Selenium may play an important protective role against COVID-19 (see Figure 5). As it is well-known, the coronavirus pandemic started in the city of Wuhan in the Hubei province of China, in December 2019. Like many Chinese provinces, such as Sichuan and Shaanxi, Hubei is a region of selenium deficiency with low selenium content in the soil [171]. Recently, it has been observed a significant association between COVID-19 cure rate and selenium status in 17 cities outside Hubei (P < 0.0001). The cure rate inside Hubei Province, of which Wuhan is the capital, was significantly lower than that in all other provinces combined [172].
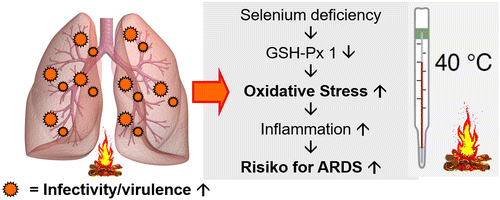
Selenium prevents viral infections from producing harmful oxidative stress. Corona- and influenza viruses increase the burden with ROS such as H2O2. Combined with the increased oxidative stress due to the reduced activity of the selenium-dependent protein glutathione peroxidases (GSH-Px) in selenium deficiency, this may lead to direct oxidative damage of the viral RNA. In the case of selenium deficiency, these mutations may turn a relative harmless influenza A virus into a considerably more aggressive type [167, 170, 173, 174]. Selenoneine is the naturally occurring selenium compound in humans. It is the selenium analogue of the sulfur-containing compound ergothioneine, a ubiquitous antioxidant found in the cells. In the form of selenoneine, selenium can inhibit angiotensin-converting enzyme (ACE) [174, 175]. Mpro is the main protease of SARS-CoV-2 and promotes the formation of its viral replication complex. As described above, the cytosolic selenoprotein GSH-Px 1 has multiple antiviral properties. It has been observed that Mpro can be inhibited by the GSH-Px 1 [177, 178]. Furthermore the influence of selenium on modulation of H2O2 induced oxidative stress on transient receptor potential melastatin 2 (TRPM2) may also reduce viral replication of SARS-CoV-2 (see Table 4) [179, 180]. In a recent, German cross-sectional study with COVID-19 patients a significant invers association between selenium status and the mortality risk was observed. Selenium was significantly higher in samples from surviving COVID patients as compared with non-survivors (serum selenium: 53.3 μg/L vs. 40.8 μg/L), recovering with time in survivors while remaining low or even declining in non-survivors [174].
The selenium status constitutes in general a risk factor for viral infections and is mostly low in patients before the infection with SARS-CoV-2. The requirement of selenium in COVID-19 disease and upon the growing inflammation increases. This increased requirement of selenium can even be reinforced through the inflammatory and hypoxic conditions stay on ICU. As the supplementation of selenium is a universally inexpensive and safe preventive measure we recommend compensate any deficiency of immune-relevant micronutrients, wherever possible after performing lab tests. This seems particularly important for the trace element selenium. Efforts should be made to achieve a target selenium level between 130 and 150 μg/L [161, 165, 172, 181].
Recommendation
Prevention
To prevent viral respiratory tract infections, elderly, adults and adolescents should take 100–200 μg selenium as sodium selenite or selenomethionine per day (approximately 2 μg selenium per kg body weight per day). Optimal preventative serum selenium levels are 130 to 150 μg/L.
Supportive treatment: hospital admission, severe course of COVID-19
- a)Initially (days 1–7): 1000 μg sodium selenite per day, in 100 mL 0.9% NaCl as a short intravenous infusion. Alternatively: 1000 μg sodium selenite per day, as a drink ampoule taken by mouth on an empty stomach for one week.
- b)Then: 300–500 μg selenium as sodium selenite daily, by mouth.
Zinc
Amongst the essential micronutrients that are necessary for normal immune system function, zinc has a key role. Zinc is present in muscle (60%), bone (30%) and other organs (10%) such as brain, kidney, liver, prostate, pancreas, skin, etc. Zinc acts a catalytic core or structural ion in more than 3000 enzymes and proteins. Daily zinc requirement of an adult is 15 mg and normal range of serum zinc concentration is 84–159 μg/dL. Zinc deficiency is common in the elderly. A study in a group of elderly European people revealed that 44% had zinc deficiency and 20% had high zinc deficiency. Zinc deficiency has been associated with thymus involution, reduced proliferation of lymphocytes, production of interleukin-2, and antibody response to T cell-dependent antigens [182, 183]. The subclinical zinc deficiency might be responsible for the high incidence of infections and degenerative pathologies related to age. For example, as zinc is involved in the synthesis of bone matrix constituents, zinc deficiency is also a risk factor for age related osteoporosis [184, 185]. Zinc supports components of the innate and adaptive immune systems, which include the three main lines of defence: epithelial barriers, cellular defences, and antibodies. A dietary zinc deficiency causes an increased susceptibility to oxidative damage of membrane fractions in several tissues. Zinc regulates the vitamin A balance via retinol binding protein (RBP). The trace element enhances not only cellular defences but also the humoral immune response. T lymphocytes responsible for cellular defence undergo a maturation process in the thymus under the influence of the hormone thymulin. This process of T cell differentiation is exclusively zinc-dependent, as only the zinc-thymulin complex is immunoactive. In zinc deficiency, the concentrations of zinc-thymulin complex in the blood are reduced and the activity of various immune cells (e.g. killer cells) is severely impaired [186]. The result is a general weakening of the body’s defences, associated with a greater susceptibility to viral infections. Zinc deficiency leads to the overproduction of pro-inflammatory mediators. Furthermore, the thymus atrophies, the number of naïve B cells falls, there is an imbalance between type 1 and type 2 T helper cells and an increase in type 17 T helper cells. In contrast, the number of regulatory T cells decreases [186, 187].
Zinc and respiratory viruses
Zinc has been shown to exhibit antiviral properties by inhibition of RNA synthesis, viral replication, DNA polymerase, reverse transcriptase, and viral proteases. Furthermore, Zinc has local antiviral effects especially in the pharyngeal region. The common cold is primarily caused by respiratory viruses that have over 100 serotypes. Among these, rhinoviruses are the most important, which are transmitted by droplet spread or smear infection (e.g. shaking hands). A body with an already weak immune system offers respiratory viruses an ideal milieu for replication. Zinc has a direct antiviral action. Numerous zinc binding sites can be found on the surface of rhinoviruses. In vitro, zinc inhibits viral replication and docking of the viruses on the mucosal receptors through which the pathogens penetrate the host cells. Recent meta-analysis of randomized double-blind, placebo-controlled trials found that high dose of zinc acetate lozenges reduced the duration of common colds symptoms by 22% (e.g. sneezing) to 54% (e.g. muscle ache), mean 42% [188].
Zinc and COVID-19
Zinc has potent immunomodulatory and antiviral properties, and can be utilized in the treatment of COVID-19 [189] (see Table 5). Zinc supplementation may favour COVID-19 treatment and enhance the efficiency of drugs such as remdesivir, dexamethasone or ribavirin. The majority of patients with COVID-19 when admitted to the hospital suffer from acute zinc deficiency [176]. Results from pre-clinical studies assume that zinc can impair replication of RNA viruses, including SARS-CoV-1, through direct inhibition of RNA-dependent RNA polymerase. Therefore, it can be hypothesized that zinc may also inhibit SARS-CoV-2 replication. Coronaviruses and influenza viruses are similarly transmitted by droplet spread. Risk groups and symptoms of COVID-19 and zinc deficiency reveal a large overlap [191]. Some studies have shown that a zinc deficiency favours the interaction of ACE-2 with SARS-CoV-2 spike protein and likewise increased zinc levels inhibit ACE-2 expression resulting in reduced viral interaction [191–193].
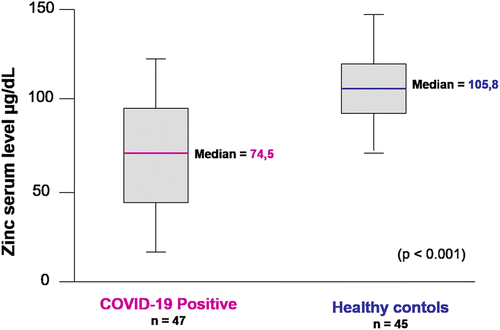
Case reports on four patients showed a significant improvement in symptomatic disease parameters after one day of high dose therapy with zinc lozenges suggesting that zinc therapy may play a role in clinical recovery [192]. A recent prospective study of fasting serum zinc levels in COVID-19 patients (n = 47), median age of 34.0 years (range 18–77 years) at the time of hospitalization revealed that COVID-19 patients had significantly lower zinc serum levels [74.5 μg/dL versus (vs) 105.8 μg/dL] in comparison to the healthy controls (n = 45) (see Figure 6) (p < 0.001). Amongst COVID-19 patients, 57.4% were found to be zinc deficient. Zinc deficient patients had a more severe disease spectrum, with a higher complication rate (70.4% vs 30.0%, p = 0.009), with an OR of 5.54. In addition, these COVID-19 patients showed an increased trend towards the development of acute respiratory distress syndrome (ARDS) (18.5% vs 0%, p = 0.06), longer hospital stays (mean 7.9 vs 5.7 days, p = 0.048), were more likely to receive corticosteroids (44.4% vs 10%, p = 0.02), and had a increased mortality (5 (18.5%) vs 0 (0%), p = 0.06) [193].
These data are corroborated by another recent published retrospective observational study with 249 COVID-19 patients. A zinc level < 50 μg/dL at the time of admission correlated significantly with a worse clinical presentation, longer time to reach stability and higher mortality [194]. The zinc and selenoprotein P status within the reference ranges indicate a high survival odd in patients with COVID-19, and assume that correcting a diagnostically proven deficit in zinc and/or selenium by a personalised supplementation may support convalescence [195].
In a recent multicenter cohort study 1006 of 3473 hospitalized patients with reverse-transcriptase-polymerase-chain-reaction (RT-PCR) positive SARS-CoV-2 infection received zinc with an ionophore. The supplementation of zinc (50 mg one or twice daily) with the ionophore hydroxychloroquine was associated with a 24% reduced risk of in-hospital mortality (HR: 0.76, 95% CI 0.60–0.96, P = 0.023) [196].
Zinc has complex immunomodulatory, antioxidant, anti-inflammatory and antiviral activities. Zinc may also inhibit RNA dependent RNA polymerase of RNA viruses such as SARS-CoV-2. Therefore zinc may reduce the risk, duration and severity of SARS-CoV-2 infections, especially in populations at high risk of zinc deficiency including people with chronic disease co-morbidities and older adults. However, there is urgent need for more clinical data about efficacy of zinc supplementation against COVID-19 [197–202].
Recommendation
Prevention
Clinical trials have confirmed the efficacy of zinc preparations in the prevention and treatment of viral respiratory tract infections. Zinc can significantly reduce the duration and severity of common colds. To prevent viral infections of the respiratory tract the elderly, adults and adolescents should supplement 10–20 mg zinc per day.
Supportive treatment: Hospital admission, severe course of COVID-19
For therapeutic effectiveness in acute infections (e.g. sore throat or runny nose), it is necessary to have a sufficiently high zinc concentration and direct contact of the zinc ions with the viral surface. When treating acute respiratory tract infections, therefore, zinc lozenges (e.g. zinc acetate or gluconate) should be sucked to allow free zinc ions to develop their inhibitory effects on the viruses.
- a)Initially (days 1–2): 20–50 mg zinc intravenously plus 7.5 g vitamin C per day; additionally: 100–150 mg zinc dived throughout the day by mouth for 7 days (e.g. zinc lozenges with zinc acetate or zinc gluconate).
- b)Then: 20–50 mg zinc per day by mouth (e.g. zinc lozenges with zinc acetate or gluconate).
Conclusion
The supplementation with micronutrients, including vitamin D and zinc is a safe, effective, and low-cost strategy to help support optimal immune function in times of respiratory tract infections with SARS-CoV-2. The application of immune-relevant micronutrients above the recommended dietary allowance (RDA), but within recommended upper safety limits, for specific micronutrients such as vitamins D and zinc is urgently warranted, especially in vulnerable groups such as the elderly. Public health officials are encouraged to promote nutritional strategies in their recommendations to improve public health, especially in vulnerable groups such as the elderly.
References
1 . Early transmission dynamics in Wuhan, china, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207.
2 . Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan. China. J Med Virol. 2020;92(4):441–7.
3 World Health Organization, editor. COVID-19 Strategy Update [Internet]. Geneva: World Health Organization; 14 April 2020. Available from: https://www.who.int/publications/i/item/covid-19-strategy-update-14-april-2020
4 . A review of micronutrients and the immune system – working in harmony to reduce the risk of infection. Nutrients. 2020;12(1):236.
5 . Immune function and micronutrient requirements change over the life course. Nutrients. 2018;10(10):1531.
6 . Nutritional modulation of immune function: analysis of evidence, mechanisms, and clinical relevance. Front Immunol. 2018;9:3160.
7 . Causes, consequences, and reversal of immune system aging. J Clin Investig. 2013;123:958–65.
8 . Vitamin C may reduce the duration of mechanical ventilation in critically ill patients: a meta-regression analysis. J Intensive Care. 2020;8:15.
9 . Vitamin C can shorten the length of stay in the ICU: A Meta-analysis. Nutrients. 2019;11(4):708.
10 . Vitamin C as a possible therapy for COVID-19. Infect Chemother. 2020;52(2):222–3.
11 . Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis. Health Technol Assess. 2019;23(2):1–44.
12 . Serum levels of vitamin C and vitamin D in a cohort of critically ill COVID-19 patients of a North American Community hospital intensive care unit in May 2020: a pilot study. Med Drug Discov. 2020;100064.
13 . Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Arch Med Res. 2020;51(5):384–7.
14 . SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses. 2020;143:110102.
15 . Vitamin D and inflammation: potential implications for severity of Covid-19. Ir Med J. 2020;113(5):81.
16 . Overview of the immune response. J Allergy Clin Immunol. 2010;125(Suppl 2):S2–23.
17 Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients. 2020;12(6):1562.
18 . Vitamins C, D and zinc: Synergistic roles in immune function and infections. Vitam Miner. 2017;6:3.
19 . Importance of dietary changes during the coronavirus pandemic: how to upgrade your immune response. Front Public Health. 2020;8:476.
20 . An overview of the immune system. Lancet. 2001;357(9270):1777–89.
21 Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. https://doi.org/10.3390/nu12040988.
22 Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax. 2015;70:617–24.
23 . Assessment of malnutrition in older persons: a focus on mini nutritional assessment. J Nutr Health Aging. 2011;15(2):87–90.
24 Nutritional status in older persons according to healthcare setting: A systematic review and meta-analysis of prevalence data using MNA®. Clin Nutr. 2016;35(6):1282–90.
25 . The final word on nutritional screening and assessment in older persons. Curr Opin Clin Nutr Metab Care. 2018;21(1):24–9.
26 . Malnutrition and ageing. Postgrad Med J. 2006;82:2–8.
27 . Malnutrition in older adults. AJN. 2018;118(3):34–41.
28 . Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82(5):1065–67.
29 . Micronutrients in the life cycle: Requirements and sufficient supply. NFS Journal. 2018;11:1–11.
30 . Protein requirements of elderly people. Dtsch Med Wochenschr. 2014;139(06):239–42.
31 . Outcomes related to nutrition screening in community living older adults: A systematic literature review. Arch Gerontol Geriatr. 2016;62:9–25.
32 Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. 2020;74(6):871–5.
33 . Important drug-micronutrient interactions: A selection for clinical practice. Crit Rev Food Sci Nutr. 2020;60(2):257–75. https://doi.org/10.1080/10408398.2018.1522613.
34 . The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2019;2020:1–4.
35 Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J. 2020;287(17):3693–702.
36 . Vitamin D deficiency. N Eng J Med. 2007;357:266–81.
37 Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30.
38 . Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S–6S.
39 . Vitamin D: Update 2013. From rickets prophylaxis to general healthcare. Dermatoendocrinol. 2013;5(3):331–47.
40 . Live longer with vitamin D? Nutrients. 2015;7(3):1871–80.
41 . Vitamin D: Die Heilkraft des Sonnenvitamins. 4th ed. Stuttgart: Wissenschaftliche Verlagsgesellschaft; 2020.
42 . The global epidemiology of vitamin D status. J Aging Res Clin Practi. 2014;3(3):148–58.
43 Low 25-hydroxyvitamin D is associated with increased mortality in female nursing home residents. J Clin Endocrinol Metab. 2012;97:E653–57.
44 Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 2016;103(4):1033–44.
45 . Age, vitamin D, and solar ultraviolet. Lancet. 1989;2(8671):1104–5.
46 . Solar UV doses of adult Americans and vitamin D 3 production. Dermato-Endocrinology. 2011;3(4):243–50.
47 . Immunologic effects of vitamin D on human health and disease. Nutrients. 2020;12(7):2097.
48 . Editorial: low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment Pharmacol Ther. 2020;51(12):1434–7.
49 Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS ONE. 2020;15(9):e0239799.
50 . Patterns of COVID-19 mortality and vitamin D: An Indonesian study. SSRN Electron J. 2020.
51 . The non-genomic actions of vitamin D. Nutrients. 2016;8:135. https://doi.org/10.3390/nu8030135.
52 . Vitamin D and influenza – prevention or therapy? Int J Mol Sci. 2018;19:2419. https://doi.org/10.3390/ijms19082419.
53 . Vitamin D and respiratory tract infections: A systematic review and meta-analysis of randomized controlled trials. PLoS One. 2013;8:e65835.
54 Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. https://doi.org/10.1136/bmj.i6583.
55 . Vitamin supplementation at the time of immunization with a cold-adapted influenza virus vaccine corrects poor mucosal antibody responses in mice deficient for vitamins A and D. Clin Vaccine Immunol. 2016;23(3):219–27.
56 The evident and the hidden factors of vitamin D status in older people during COVID-19 pandemic. Nutrire. 2021;46(1):1. https://doi.org/10.1186/s41110-020-00131-3.
57 . SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15(9):e0239252.
58 . Impact of serum 25(OH) vitamin D level on mortality in patients with COVID-19 in Turkey. J Nutr Health Aging. 2020;5:1–8. https://doi.org/10.1007/s12603-020-1479-0.
59 . Human antimicrobial peptides as therapeutics for viral infections. Viruses. 2019;11(8):704.
60 COVID-19 and Vitamin D: A lesson from the skin. Exp Dermatol. 2020. https://doi.org/10.1111/exd.14170.
61 Reply to Jakovac and to Rocha et al.: Can vitamin D prevent or manage COVID-19 illness? Am J Physiol Endocrinol Metab. 2020;319(2):E455–7.
62 . Deficiency: effects on oxidative stress, epigenetics, gene regulation, and aging. Biology. 2019;8(2):30.
63 Vitamin D receptor inhibits NLRP3 activation by impeding Ist BRCC3-mediated deubiquitination. Front Immunol. 2019;10:2783. https://doi.org/10.3389/fimmu.2019.02783.
64 . Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol Med Rep. 2017;16(5):7432–8.
65 . Vitamin D deficiency and co-morbidities in COVID-19 patients – A fatal relationship? NFS Journal. 2020;20:10–21.
66 . The effect of various doses of oral vitamin D 3 supplementation on gut microbiota in healthy adults: a randomized, double-blinded, dose-response study. Anticancer Res. 2020;40(1):551–6.
67 . A narrative role of vitamin D and its receptor: with current evidence on the gastric tissues. Int J Mol Sci. 2019;20(15):3832.
68 . Spermidine: A novel autophagy inducer and longevity elixir. Autophagy. 2010;6(1):160–2.
69 Vitamin D3 induces autophagy in human monocytes/macorphages via cathelicidin. Cell Host Microbe. 2009;6(3):231–43.
70 Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study). Postgrad Med J. 2020; [Epub ahead of print].
71 . Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci Rep. 2020;10:20191. https://doi.org/10.1038/s41598-020-77093-z.
72 . Vitamin D and survival in COVID-19 patients: A quasi-experimental study. J Steroid Biochem Mol Biol. 2020;204:105771. https://doi.org/10.1016/j.jsbmb.2020.105771.
73 . Vitamin D on Prevention and Treatment of COVID-19. ClincialTrials [Internet]. April 3, 2020 [cited 2020 Nov 29]. Available from: https://Clinicaltrials.gov/ct2/show/NCT04334005.
74 . COVID-19 and vitamin D - Is there a link and an opportunity for intervention? Am J Physiol Endocrinol Metab. 2020;318(5):E589.
75 . Vitamin D supplementation: a potential approach for COVID-19 therapeutics? Front Immunol. 2020;11:1523.
76 . Disassociation of vitamin D’s calcemic activity and non-calcemic genomic activity and individual responsiveness: a randomized controlled double-blind clinical trial. Sci Rep. 2019;9:17685. https://doi.org/10.1038/s41598-019-53864-1.
77 . Micronutrients [Internet]. [cited 2020 Oct 31]. Available from: https://www.who.int/nutrition/topics/vad/en/
78 . Dietary surveys indicate vitamin intakes below recommendations are common in representative Western countries. Br J Nutr. 2012;108(4):692–8.
79 . Vitamin A deficiency in elderly attending the Health Family Programme in Camaragibe, PE, Brazil. Arch Latinoam Nutr. 2007;57(3):213–8.
80 Study on vitamin A nutritional status of Chinese urban elderly residents in 2010–2012. Zhonghua Yu Fang Yi Xue Za Zhi. 2017;51(2):121–4.
81 . Serum retinol concentrations of Chinese rural elderly residents in 2010–2012. Wei Sheng Yan Jiu. 2017;46(3):356–60.
82 The vitamin A derivative all-trans retinoic acid repairs amyloid-β-induced double-strand breaks in neural cells and in the murine neocortex. Neural Plasticity. 2016;3707406. https://doi.org/10.1155/2016/3707406.
83 . The challenge to reach nutritional adequacy for vitamin A: b-carotene bioavailability and conversion – evidence in humans. Am J Clin Nutr. 2012;96:1193S–203S.
84 . Bioconversion of dietary provitamin A carotenoids to vitamin A in humans. Am J Clin Nutr. 2010;91(5):1468S–1473S.
85 . Single nucleotide polymorphisms upstream from the β-carotene 15,15’-monoxygenase gene influence provitamin A conversion efficiency in female volunteers. J Nutr. 2012;142(1):161S–165S.
86 Two common single nucleotide polymorphisms in the gene encoding beta-carotene 15,15’-monoxygenase alter beta-carotene metabolism in female volunteers. FASEB J. 2009;23(4):1041–53.
87 . Concentrations of selected carotenoids and vitamin A in human liver, kidney and lung tissue. J Nutr. 1991;121(10):1613–21.
88 . Retinoid induction of alveolar regeneration: from mice to man? Thorax. 2009;64(5):451–7.
89 . Assessing Vitamin A Status: Past, Present and Future. J Nutr. 2004;134(1):290S–293S.
90 . A low molar ratio of retinol binding protein to transthyretin indicates vitamin A deficiency during inflammation: studies in rats and a posterior analysis of vitamin A-supplemented children with measles. J Nutr. 1998;128(10):1681–7.
91 Adjusting retinol-binding protein concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106(Suppl 1):390S–401S.
92 Rapid diagnostic testing platform for iron and vitamin A deficiency. PNAS. 2017;114(51):13513–8.
93 . Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8(9):685–98.
94 . Molecular basis for vitamin A uptake and storage in vertebrates. Nutrients. 2016;8(11):676.
95 . The contribution of vitamin A to public health. FASEB J. 1996;10:1040–148.
96 Consequences of vitamin A deficiency: immunoglobulin dysregulation, squamous cell metaplasia, infectious disease, and death. Int J Mol Sci. 2020;21:5570. https://doi.org/10.3390/ijms21155570.
97 Baseline serum vitamin A and D levels determine benefit of oral vitamin A & D supplements to humoral immune responses following pediatric influenza vaccination. Viruses. 2020;11(10):907.
98 . Alcohol and aldehyde dehydrogenases: retinoid metabolic effects in mouse knockout models. Biochim Biophys Acta. 2012;1821(1):198–205.
99 . Role of vitamin A in the immune system. J Clin Med. 2018;7:258. https://doi.org/10.3390/jcm7090258.
100 . The vitamin A story - lifting the shadow of the death. World Rev Nutr Diet. 2012;104:132–50.
101 . Vitamin A, immunity, and infection. Clin Infect Dis. 1994;19(3):489–99.
102 . Vitamin A and immunity to viral, bacterial and protozoan infections. Proc Nutr Soc. 1999;58(3):719–27.
103 Impact of vitamin A and carotenoids on the risk of tuberculosis progression. Clin Infect Dis. 2017;65(6):900–9.
104 . Low plasma levels of cholecalciferol and 13-cis-retinoic acid in tuberculosis: implications in host-based chemotherapy. Nutrition. 2013;29(10):1245–51.
105 Vitamin A deficiency impairs mucin expression and suppresses the mucosal immune function of the respiratory tract in chicks. PLoS One. 2015;10(9):e0139131.
106 . Retinoic acid differentially regulates the migration of innate lymphoid cell subsets to the gut. Immunity. 2015;43:107–19.
107 . The effect of vitamin A on epithelial integrity. Proc Nutr Soc. 1999;58(2):289–93.
108 Reciprocal th17 and regulatory t cell differentiation mediated by retinoic acid. Science. 2007;317(5835):256–60.
109 . Effects of vitamin A supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev. 2005;18(3):446–64.
110 . Modulation of T Cell and innate immune responses by retinoic acid. J Immunol. 2014;192:2953–8.
111 . Retinoic acid-dependent regulation of immune responses by dendritic cells and macrophages. Semin Immunol. 2009;21(1):22–7.
112 . Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. Cochrane Database Syst Rev. 2017;3(3):CD008524. https://doi.org/1002/14651858.CD008524.pub3.
113 Oxidative stress and inflammation in COVID-19-associated sepsis: the potential role of anti-oxidant therapy in avoiding disease progression. Antioxidants. 2020;9:936. https://doi.org/10.3390/antiox9100936.
114 . Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355–62.
115 . Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708.
116 . Vitamin A as an anti-inflammatory agent. Proc Nutr Soc. 2002;61(3):397–400.
117 . Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–92.
118 . Metabolic effects of inflammation on vitamin A and carotenoids in humans and animal models. Adv Nutr. 2017;8:197–212.
119 Revealing the targets and mechanisms of vitamin A in the treatment of COVID-19. Aging. 2020;12(15):15784–96.
120 . Global vitamin C status and prevalence of deficiency: a cause for concern? Nutrients. 2020;12(7):2008.
121 . Vitamin C deficiency in elderly hospitalized patients. Am J Med. 2001;111(6):502.
122 . Effects of vitamin C supplementation on blood pressure: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;95(5):1079–88.
123 Vitamin C pharmacokinetics in critically ill patients: a randomized trial of four iv regimens. Chest. 2018;153(6):1368–77.
124 Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. PNAS. 1996;93(8):3704–9.
125 . A new recommended dietary allowance of vitamin C for healthy young women. PNAS. 2001;98(17):9842–6.
126 . Authors’ perspective: What is the optimum intake of vitamin C in humans? Crit Rev Food Sci Nutr. 2012;52(9):815–29.
127 . Discrepancies in global vitamin C recommendations: a review of RDA criteria and underlying health perspectives. Crit Rev Food Sci Nutr. 2020;1–14. https://doi.org/126.1080/10408398.2020.1744513
128 . Immunomodulatory and Antimicrobial Effects of Vitamin C. Eur J Microbiol Immunol (Bp). 2019;9(3):73–9.
129 . The antiviral properties of vitamin C. Expert Rev Anti Infect Ther. 2020;18(2):99–101.
130 . Emerging evidence on neutrophil motility supporting its usefulness to define vitamin C intake requirements. Nutrients. 2017;9:503. https://doi.org/10.3390/nu9050503.
131 . Effects of different ascorbic acid doses on the mortality and critically ill patients: a meta-analysis. Ann Intensive Care. 2019;9(1):58. https://doi.org/10.1186/s13613-019-0532-9.
132 . Vitamin C and SARS coronavirus. J Antimicrob Chemother. 2003;52(6):104–50.
133 . Vitamin C and immune function. Nutrients. 2017;9:1211. https://doi.org/10.3390/nu9111211.
134 Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am J Physiol Lung Cell Mol Physiol. 2012;303:L20–32.
135 . Possible application of high-dose vitamin C in the prevention and therapy of coronavirus infection. J Glob Antimicrob Resist. 2020;23:256–62.
136 . The cytokine storm and COVID-19. J Med Virol. 2020;1–7. https://doi.org/10.1002/jmv.26232
137 Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56.
138 . Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–39.
139 . SARS-CoV-2 (Severe acute respiratory syndrome coronavirus 2) pandemic and neurology. DGNeurologie. 2020;3(4):273–4.
140 . Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: Lessons from SARS and MERS, and potential therapeutic interventions. Life Sciences. 2020;257:118102.
141 . Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine. 2004;27(4–5):101–6.
142 . Vitamin C inhibits granulocyte macrophage-colony-stimulating factor-induced signalling pathways. Blood. 2002;99(9):3205–12.
143 Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediators Inflamm. 2014;2014:426740.
144 Ascorbic acid promotes proliferation of natural killer cell populations in culture systems applicable for natural killer cell therapy. Cytotherapy. 2015;17(5):613–20.
145 . The clinical effects of vitamin C supplementation in elderly hospitalised patients with acute respiratory infections. Int J Vit Nutr Res. 1994;64(3):212–9.
146 . Vitamin C can shorten the length of stay in the ICU: a meta-analysis. Nutrients. 2019;11. https://doi.org/10.3390/nu11040708.
147 . The role of vitamin C as adjuvant therapy in COVID-19. Cureus. 2020;12(11):e11779.
148 Extra dose of vitamin c based on a daily supplementation shortens the common cold: a meta-analysis of 9 randomized controlled trials. Biomed Res Int. 2018;2018:1837634.
149 . Vitamin C administration in the critically ill: a summary of recent meta-analyses. Crit Care. 2019;23:265. https://doi.org/10.1186/s13054-019-2538-y.
150 . Vitamin C Infusion for the Treatment of Severe 2019-nCoV Infected Pneumonia: a Prospective Randomized Clinical Trial. [Internet]. 2020 Feb 11. Available from: https://clinicaltrials.gov/ct2/show/NCT04264533.
151 . Shanghai Coronavirus Disease Clinical Treatment Expert Group. Direct Translation of Shanghai Management Giudeline for Covid-19. Cin J Infect Dis. 2020;38. https://doi.org/10.3760/cma.j.issn.1000-6680.2020.0016.
152 Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA. 2019;322(13):1261–70.
153 . Quercetin and vitamin C: An experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19). Front Immunol. 2020;11:1451.
154 . Intravenous high-dose vitamin C for the treatment of severe COVID-19: study protocol for a multicentre randomised controlled trial. BMJ Open. 2020;10:e039519.
155 . Critical care management of adults with community-acquired severe respiratory viral infection. Intensive Care Med. 2020;46(2):315–28.
156 . The Emerging Role of Vitamin C in the Prevention and Treatment of COVID-19. Nutrients. 2020;12(11):3286.
157 , Zinc, Vitamin D and Vitamin C: Perspectives for COVID-19 With a Focus on Physical Tissue Barrier Integrity. Front Nutr. 2020;7:606398. https://doi.org/10.3389/fnut.2020.606398.
158 . COVID-19 Treatment Guidelines, Vitamin C [Internet]. 2020 Nov 3. Available from: https://www.covid19treatmentguidelines.nih.gov/adjunctive-therapy/vitamin-c.
159 . Selenoproteins: molecular pathways and physiological roles. Physiol Rev. 2014;94(3):739–77.
160 . Selenoprotein-P deficiency predicts cardiovascular disease and death. Nutrients. 2019;11:1852. https://doi.org/10.3390/nu11081852.
161 Selenium status is associated with colorectal cancer risk in the European prospective investigation of cancer and nutrition cohort. Int J Cancer. 2015;136(5):1149–61.
162 . Selenium and human health. Lancet. 2012;379:1256–68.
163 Selenium deficiency risk predicted to increase under future climate change. Proc Natl Acad Sci USA. 2017;114(11):2848–53.
164 . The Role of Selenium in Inflammation and Immunity: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid Redox Signal. 2012;16(7):705–43.
165 . The other view: The trace element selenium as a micronutrient in thyroid disease, diabetes, and beyond. Hormones (Athens). 2020;19(1):15–24.
166 . Micronutirents in Oncological Intervention. Nutrients. 2016;8:3. https://doi.org/10.3390/nu8030163.
167 . Review: Micronutrient Selenium Deficiency Influences Evolution of Some Viral Infectious Diseases. Biol Trace Elem Res. 2011;143:1325–36.
168 . Reactive oxygen and nitrogen species during viral infections. Free Radic Res. 2014;48(10):1163–9.
169 . Selenium, selenoproteins and viral infection. Nutrients. 2019;11(9):2101. https://doi.org/10.3390/nu11092101.
170 . Selenium and selenoproteins in prostanoid metabolism and immunity. Crit Rev Biochem Mol Biol. 2019;54(6):484–516. https://doi.org/10.1080/10409238.2020.1717430.
171 . The influence of selenium on immune responses. Mol Nutr Food Res. 2008;52:1273–80.
172 . Host nutritional status: the neglected virulence factor. Trends Microbiol. 2004;12:417–23.
173 Selenium distribution in the Chinese environment and its relationship with human health: a review. Environ Int. 2018;112:294–309.
174 . Association between regional selenium status and reported outcome of COVID-19 cases in China. Am J Clin Nutr. 2020;111(6):1297–9.
175 Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients. 2020;12:2098. https://doi.org/10.3390/nu12072098.
176 . Selenium supplementation in the prevention of coronavirus infections (COVID-19). Medical Hypotheses. 2020;143:109878.
177 . Identification of a novel selenium containing compound, selenoneine, as the predominant chemical form of organic selenium in the blood of bluefin tuna. J Biol Chem. 2010;285:18134–8.
178 . Inhibition of angiotensin-converting enzyme by selenoneine. Fisheries Science. 2019;85:731–6.
179 Structure of M(pro) from COVID-19 virus and discovery of its inhibitors. Nature. 2020;582:289–93.
180 . A role for selenium-dependent GPX1 in SARS-CoV-2 virulence. Am J Clin Nutr. 2020;112(2):447–8.
181 . TRPM2: A multifunctional ion channel for calcium signalling. J Physiol. 2011;589(7):1515–25.
182 . Selenium supplementation can relieve the clinical complications of covid-19 and other similar viral infections. Int J Vitam Nutr Res. 2020;9:1–3.
183 . Zinc – the underestimated element. Med Monatsschr Pharm. 2020;43:149–58.
184 . Zinc Biochemistry: From a Single Zinc Enzyme to a Key Element of Life. Adv Nutr. 2013;4:82–91.
185 . COVID-19 and nutriceutical therapies, especially using zinc to supplement antimicrobials. Inflammopharmacol. 2020;1–5. https://doi.org/10.1007/s10787-020-00774-8.
186 . Zinc, aging, and immunosenescence: an overview. Pathobiol Aging Age Relat Dis. 2015;5:10.
187 . The Role of Zinc in Antiviral Immunity. Adv Nutr. 2019;10(4):696–710.
188 . Zinc in infection and inflammation. Nutrients. 2017;9(6):624.
189 . Zn(2 +) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLOS Pathog. 2010;6(11):e1001176.
190 . The effectiveness of zinc acetate lozenges on various common cold symptoms: a meta-analysis. BMC Fam Pract. 2015;16:24. https://doi.org/10.1186/s12875-015-0237-6.
191 . Zinc acetate lozenges for the treatment of the common cold: a randomised controlled trial. BMJ Open. 2020;10(1):e031662. https://doi.org/10.1136/bmjopen-2019-031662.
192 . The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis. Front Immunol. 2020;11:1712.
193 . Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45.
194 . ACE2 receptor polymorphism: susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J Microbiol Immunol Infect. 2020;53(3):425–35.
195 . Case Report Treatment of SARS-CoV-2 with high dose oral zinc salts: A report on four patients. Int J Infect Dis. 2020;99:307–9.
196 COVID-19: Poor outcomes in patients with zinc deficiency. Int J Infect Dis. 2020;100:343–9.
197 Low zinc levels at clinical admission associates with poor outcomes in COVID-19. medRxiv preprint. 2020. https://doi.org/10.1101/2020.10.07.20208645
198 Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker. Redox Biol. 2021;38:101764.
199 Treatment with zinc is associated with reduced in-hospital mortality among COVID-19 patients: a multi-center cohort study. Res Sq, Preprint. 2020. https://doi.org/10.21203/rs.3.rs-94509/v1.
200 Zinc for the prevention and treatment of SARS-CoV-2 and other acute viral respiratory infections: a rapid review. Adv Integr Med. 2020;7(4):252–60.
201 Zinc and COVID-19: Basis of Current Clinical Trials. Biol Trace Elem Res. 2020. https://doi.org/10.1007/s12011-020-02437-9.
202 . Benefits and risks of zinc for adults during covid-19: rapid systematic review and meta-analysis of randomised controlled trials. medRxiv preprint. 2020. https://doi.org/10.1101/2020.11.02.20220038.