Provisional focal stenting of complex femoropopliteal lesions using the Multi-LOC multiple stent delivery system – 12-month results from the LOCOMOTIVE EXTENDED study
Abstract
Summary:Background: This study aimed to evaluate a Multiple Stent Delivery System for provisional focal stenting of the femoropopliteal artery. Patient and methods: The LOCOMOTIVE EXTENDED study (Multi-LOC for flOw liMiting Outcomes after plain old balloon angioplasty and/or drug-coated balloon Treatment in the infrainguinal position with the objectIVE to implant multiple stent segments) is a prospective, single-arm, multicentre observational study. The Multi-LOC Multiple Stent Delivery System (B.Braun, Melsungen, Germany) was used for provisional focal stenting of the femoropopliteal artery. We enrolled 357 patients with 449 femoropopliteal lesions; all had flow-limiting dissections or recoil following angioplasty. Eligibility included Rutherford classification 2 to 5 with a de novo or non-stented restenotic femoropopliteal lesion undergoing plain balloon or drug-coated balloon angioplasty. The 6- and 12-month efficacy endpoints encompassed target lesion revascularisation and primary patency rates. Results: The mean patient age was 71 ± 10 years. The mean lesion length was 16.0 ± 9.7 cm; 44.5% were TASC II C/D lesions and 31.4% were chronic total occlusions. By operator choice, 45% of the patients underwent drug-coated balloon angioplasty. On average, 4.0 stents (each 13 mm long) were placed in each lesion, resulting in a scaffolding proportion of 56% of the total lesion length with a technical success rate of 98.3%. At 6 and 12 months, the freedom from clinically driven target lesion revascularisation was 95.5% and 88.7% and the primary patency rates were 88.7% and 82.3%, respectively. At 12 months, significant improvements were noted in Rutherford categories and ankle-brachial indices. In multiple regression analyses, both diabetes mellitus and no distal run-off vessel showed a trend toward worse TLR, while other factors such as DCB predilation or the lesion length were not predictive. Conclusions: The LOCOMOTIVE EXTENDED study demonstrated the safety and efficacy of the Multi-LOC stent system for focal provisional stenting of complex femoropopliteal lesions.
Introduction
In peripheral arterial interventions, the femoropopliteal (FP) segment remains one of the most challenging regions in the endovascular treatment field. Numerous mechanical stressors like flexion, extension, elongation and compression, and often “bone-like” high plaque burden make the FP arteries difficult to deal with [1, 2]. Different strategies ranging from long-segment scaffolding to non-stent-based solutions (e.g., drug-coated-balloons, scoring balloons, and atherectomy) improved the primary patency (PP) and reduced the target lesion revascularisation (TLR) rates; however, often, the long-term results were unsatisfying or the interventional techniques created new problems or concerns. Consequently, it became clear that stent usage is often indispensable, especially in long and calcified lesions. Therefore, the “go-between” the “leaving nothing behind” and the “full-metal jackets” approach is considered a reasonable compromise [3].
Previously, we reported proof of concept in an animal model [4] and the clinical outcomes from the first-in-man registry trial demonstrating the safety and efficacy of the Multiple Stent Delivery System (MSDS) with favourable TLR and PP rates in claudicants and critical limb ischaemia patients [5, 6]. The aim of this study was to assess the safety and efficacy of a peripheral MSDS designed for focal stenting to treat FP lesions after unsatisfying results with plain old balloon angioplasty (POBA) or drug-coated balloon (DCB) dilatations in complex lesions on a large, international scale.
Patients and methods
Study design and setting
The LOCOMOTIVE EXTENDED study was a prospective, single-arm, multicentre, observational study (ClinicalTrials.gov Identifier: NCT02900274) conducted in high-volume European interventional vascular centres.
Period of recruitment and eligibility criteria
Patients were recruited from September 2016 to December 2018. Eligibility included Rutherford classification 2 to 5 with a de novo or non-stented restenotic FP lesion undergoing POBA or DCB angioplasty after provisional focal stenting due to flow-limiting dissections or recoil at the discretion of the interventionalist. The study protocol allowed for inclusion of all lesions in the superficial femoral and popliteal artery as long as the reference vessel diameter was between 4.0 mm and 7.0 mm and the lesion length considered suitable for the release of at least two Multi-LOC stents with a minimum inter-stent distance of 1 cm. The study protocol had no specific exclusion criteria.
Endpoints and follow-up
The primary endpoint was the all-cause TLR rate at 6 months. A secondary endpoint was the TLR rate at 12 months. Moreover, PP rates determined with duplex ultrasonography (defined as peak systolic velocity index greater than 2.4 at the target lesion) at 6 and 12 months were also included. Other secondary endpoints were the ankle brachial index and Rutherford category shift (difference between 12 months as com-pared to baseline). Mortality, vascular treatment outside target lesion, and all adverse events including minor and major amputations were recorded with a dedicated data capture system. Additional details of the study device are provided in a previous publication [4]. Briefly, the stent device carries six individual non-helical nitinol stents premounted on a single-hand system with a highly flexible braided outer sheath, thus forming the commercially available VascuFlex® Multi-LOC stent system (B.Braun Melsungen AG, Germany). The device is 6F compatible and available in shaft lengths of 80 cm and 130 cm. The 13-mm long stents are available in four different nominal diameters (5 mm, 6 mm, 7 mm, 8 mm).
Comedication
Dual antiplatelet therapy was recommended for at least 4 weeks, followed by acetylsalicylic acid or clopidogrel monotherapy and/or an anticoagulant, if medically necessary due to comorbidities.
Ethics
The LOCOMOTIVE EXTEND registry study was approved by the local ethics committee at each study site. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation guideline. All patients gave written informed consent.
Statistical analysis
The Pearson Chi2 test was used to analyse dichotomous variables. Moreover, the Mann-Whitney U test was used to analyse continuous variables, in case the Shapiro-Wilk test revealed a strong deviation from normal distribution. Otherwise, the unpaired t-test was utilised. The paired t-test was applied for Rutherford classes per patient at different time points. For measurements of ankle-brachial indices, the repeated measures analysis of variance was utilised. Logistic regression and Cox-regression models were used to study predictors for TLR using various patient demographic, lesion, morphological, and procedural attributes. All statistical analyses were performed with SPSS version 24 (IBM, Munich, Germany).
Results
Patient demographics
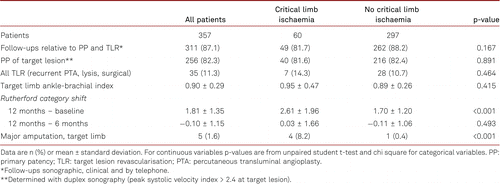
A total of 357 patients at 18 clinical sites in five European countries were included into the study. Among the enrolled patients, 313 (87.7%) completed the 12-month follow-up, and were evaluable for the primary efficacy endpoints (duplex-derived restenosis and clinically driven TLR) prior to the end of the 12-month period. Baseline demographic and clinical characteristics are summarised in Table I. The mean patient age was 71.2 ± 9.7 years. Claudication (Rutherford category 2 or 3) was noted in 82.2% of the patients and critical limb ischaemia (Rutherford category 4 or 5) in 16.8%. Approximately 49.0% of the patients had diabetes mellitus and 44.8% were current smokers. Frequent comorbidities were hypertension (87.4%), hypercholesteraemia (72.0%), and chronic kidney disease (18.8%).
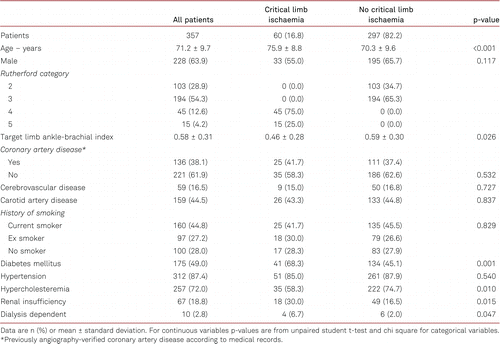
Lesion morphology
A total of 449 FP lesions were treated. The mean lesion length was 16.0 ± 9.7 cm. Table II summarises the target lesion morphology; 44.5% were TASC II C/D lesions and 31.4% were total occlusions. Angiographic calcification was present in 85.7% of all target lesions and 29.4% had a poor crural (single-vessel or no vessel) run-off.
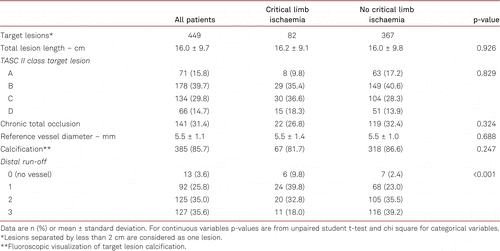
Procedure
Procedural data and device characteristics are summarised in Table III. By operator choice, more lesions were predilated with POBA (54.1%) than with DCB angioplasty (45.0%). Four patients had lesions that were not predilated (protocol violation). Per lesion, 4.0 ± 2.0 stent segments (each 13 mm long) were placed, resulting in a scaffolding proportion of 56% of the total lesion length. Reason for provisional stenting of the target lesions were flow-limiting dissection (28.1%), stenotic recoil (26.9%), and more frequently both (45.0%). The technical success rate to implant all stent segments was 98.3%.
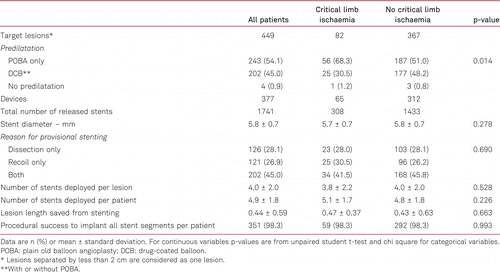
Clinical outcomes
At 6 months, the freedom from clinically driven TLR was 95.5% and the PP rate was 88.7%. The PP rate at 12 months was 82.3% in the overall cohort. At 12 months, the freedom from clinically driven TLR was 88.7% in the overall cohort, with no significant difference between claudicants and critical limb ischaemia patients in the regression analysis (log rank p = 0.325; electronic supplementary material [ESM] 1).
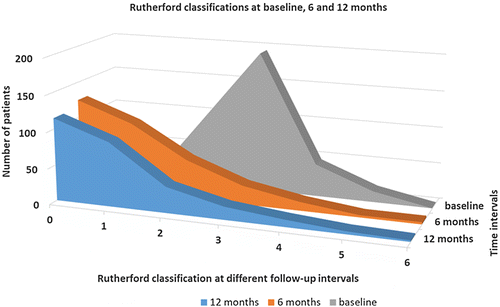
The mean Rutherford category significantly improved from 2.9 ± 0.7 (range 2–5 according to the inclusion criteria) at baseline to 1.0 ± 1.2 after 12 months (p = 0.008; Figure 1). Of note, we found no change in the Rutherford category between the 6-month and 12-month follow-ups (p = 0.493). Within one year there were five major amputations at the target leg, four of them in patients suffering from critical limb ischaemia and one in patients with former claudication (p < 0.001). Furthermore, significant improvements were noted in the ankle-brachial indices, increasing from a mean of 0.58 ± 0.31 at baseline to 0.90 ± 0.29 after 12 months (p < 0.001). The main clinical outcome data are summarized in Table IV. Additional data can be found in the ESM 2.
Predictors of TLR
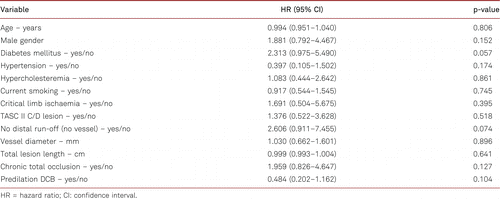
In a multiple regression analysis, both diabetes mellitus and no distal run-off vessel showed a trend toward worse TLR, while other factors such as DCB predilation or the lesion length were not predictive. These results were consistent in the Cox regression analysis (Table V) and the logistic regression analysis (ESM 3).
Discussion
The prospective multicentre LOCOMOTIVE EXTENDED study demonstrated that in a cohort composed exclusively of patients with suboptimal angiographic results after POBA or DCB dilatation due to flow-limiting dissections or recoil, the use of Multi-LOC spot stents yields a favourable freedom from TLR (95.5% and 88.7%) and PP (88.7% and 82.3%) after 6 and 12 months, respectively. The significant improvements in clinical outcome and haemodynamics confirmed the promising results obtained from earlier studies of the MSDS [5, 6]. While the rather small LOCOMOTIVE study described the first clinical experience with the Multi-LOC device, the LOCOMOTIVE EXTENDED study was conducted on a large, international scale (18 clinical sites in five European countries) and is therefore more representative for the “real-world” situation. The number of 357 patients with 449 femoropopliteal lesions allowed further statistical analyses including the impact of different variables on the outcome in multivariate analysis models. The LOCOMOTIVE EXTENDED study is the only study providing a defined “spot” stent length of 13 mm. In contrast, current definitions of spot stenting in the literature do not set predefined limits to the length of a single “spot” stent or to the stent-to-lesion length ratio. In this regard, the technique of focal stenting has as many names as faces, e.g. spot stenting, selective stenting, and the “As Less As Reasonably Achievable Strategy”. The common denominator of these techniques being the stented length is shorter than the overall treated vessel length/lesion length with an incomplete lesion coverage. Consequently, besides the differences in patients and lesions, the interventional procedures vary markedly between different FP spot stenting studies, and the results are heterogeneous.
In a recent retrospective propensity-matched analysis, Tomoi et al. [7] concluded that PP rates at 3 years were significantly lower with spot stenting than with full coverage stenting for FP lesions. However, an average number of 1.2 implants covering a mean lesion length of 13.5 cm represents rather incomplete lesion coverage using medium-length stents than “spot” stents. Nevertheless, the interaction analysis suggested that spot stenting might be suited for more complex FP lesions (i.e., chronic total occlusion lesions, proximal superficial femoral artery lesion, or lesion length of at least 138 mm). While the morphological complexity of the lesions in the study of Tomoi was slightly higher than that in our study, our cohort included 16.8% of patients with critical limb ischaemia, while Tomoi et al. excluded patient with ischaemic lesions.
In contrast, the recently published randomised controlled PARADE trial [8] (Primary Long Full Coverage Stenting versus Primary Short Spot Stenting for Long Femoropopliteal Artery Disease) showed a higher PP (86.1% vs. 72.7%) and TLR-free survival (94.2% vs. 82.5%) at 12 months in the spot stenting group. However, the study was discontinued (after only 49 patients had been enrolled within 12 months) and was therefore underpowered yielding no statistically different clinical results between treatment groups. Not far from Tomoi et al.’s analysis, an average number of 1.2 implants covered a mean lesion segment of 11.6 cm of a total mean lesion length of 24.5 cm. Notably, in a previous retrospective study, Hong et al. [9] reported a significantly higher PP rate with short stenting than with long stenting, following an intentional subintimal approach for long FP chronic total occlusions with a mean length of 25 cm.
In the studies of both Ko et al. and Tomoi et al., an average of 1.2 stents per lesion were placed, compared to 4.0 stents per lesion in the LOCOMOTIVE EXTENDED study. With a comparable scaffolding proportion of the total lesion length (47% in the PARADE TRIAL, 56% in our study, 62% in Tomoi et al.’s study), our spot stenting strategy is the most consistent in our biomechanical understanding of the FP artery, the deformation of which changes in the presence of a stent, depending on the stent length [10].
In our registry, diabetes mellitus and absence of distal run-off were both borderline predictors for an increased risk of TLR. The inconsistent data situation of endogenous factors such as diabetes mellitus on FP restenosis [11] reflects the multifactorial aetiology involving diverse endogenous, lesion- and procedure-related risk factors of restenosis [12]. Our analysis trends towards the results of previous studies reporting higher restenosis rates in patients with diabetes mellitus in long and short de novo FP lesions [13, 14]. However, patterns of restenosis after Multi-LOC spot stenting are not comparable with those after long-segment stent-based interventions [15], and in several randomised controlled trials, the correlation between restenosis and TLR is weak [16].
The lack of crural vessel run-off tended to be predictive of TLR, but it was not significant in our study, likely due to the small sample size. In a study by Lin et al. [17] investigating the occurrence of edge stenosis after covered stenting for long superficial femoral artery occlusive disease, the adequacy of distal run-off emerged as the sole predictor of edge stenosis. However, the mechanism of edge stenosis might differ from the pattern of restenosis after ML stenting [15]. Since the absence of a native crural artery in the context of FP interventions reflects the presence of a multivessel peripheral artery occlusive disease, these patients suffer from higher atherosclerotic burden.
We could not identify lesion-related nor procedure-related factors for TLR in our cohort. Neither the long lesion length nor the small diameter of the vessel or the presence of a total occlusion was associated with restenosis. These results differ from the data derived from randomised controlled trials and large registry studies using long-segment stenting [18, 19] and might be an effect of the study’s eligibility criteria (lesions with stenotic recoil and flow-limiting dissections after predilation) and the operators’ free choice of treatment modalities (balloon type, dilation time, and focal stenting instead of long stenting). In particular, predilation with DCB was not predictive of a lower risk of TLR. This study was not powered to detect differences in balloon type used, and the observed differences could be due to various factors, including operator bias for balloon selection according to patient or lesion characteristics. Finally, the efficacy of DCBs combined with Multi-LOC stents is currently unknown. Recently, a DCB-supported stent strategy using an interwoven stent did not improve the PP rate compared to the stent only strategy [20].
Overall, the lesions in our study were rather complex and required provisional stenting after predilation. As leg flexion is associated with significant stent bending and shortening [21] in addition to the fact that the deformation characteristics of the FP segment change in the presence of a stent in a stent length-dependent manner, the shorter the stent, lesser could be the dependence of TLR on morphological lesion characteristics. Notably, Tomoi et al. [7], who found an overall significantly lower PP rate in the spot stenting group than in the FCS group, co-stated the noninferiority of spot stenting in lesion lengths ≥ 138 mm and chronic total occlusions. Moreover, in the PARADE trial [8], the total stented length was an independent predictor of restenosis, although the investigators initially hypothesised that long full coverage stenting would be superior to spot stenting in a primary stenting approach.
The optimal stenting strategy for complex FP artery lesions remains undefined. Overall, the positive outcomes of the LOCOMOTIVE EXTENDED study provide evidence that the Multi-LOC stent system is effective and safe in a real-world setting. Together with the latest FP spot stenting studies across a wide range of spot stenting definitions and a diverse range of lesion complexity, the spot stenting technique offers a promising, perhaps superior in our view, alternative to long-segment stenting when scaffolding is needed. Unlike low radial force supports, suitable to facilitate the apposition of dissection flaps in lesions with predominantly (65%) mild to no calcification where low outward force is sufficient to keep the lumen open [22], spot stenting allows stabilisation of both flow-limiting dissections and stenotic recoil after angioplasty of complex FP lesions. Furthermore, following focal stenting with the Multi-LOC stent delivery system, stent-related restenosis and stent fractures seem to play a minor to non-existant role [4, 15].
While adequately powered studies with head-to-head randomisation between stenting strategies (spot stenting with “short” stents versus full metal jackets) will be the key for gaining insights in the future of focal FP stenting, the efficacy of spot stenting could possibly be further increased by combining it with additional preparation techniques, such as vessel scoring or debulking.
Limitations
This study has some limitations. The LOCOMOTIVE EXTENDED study is an “all-comer” post-market clinical follow-up study without a control group. The type of balloon used for predilation was at the discretion of the operator, and the reasons why spot stenting (instead of long stenting) was chosen for FP lesions were not assessed. As procedural routines in vascular centres vary, this may have introduced bias. However, with the enrolment from multiple centres, this observational study represents a rather real-world setting. Furthermore, lesions in four patients were not predilated, thereby constituting a protocol violation. Finally, there was no adjudication by an independent core laboratory.
Conclusions
The multiple stent delivery system Multi-LOC provides promising results concerning TLR and PP at 12 months. The LOCOMOTIVE EXTENDED study results show that the use of the Multi-LOC stent system is both safe and effective for provisional repair of flow-limiting dissections or recoil following POBA and DCB angioplasty of the FP artery. Randomized trials are needed to compare the focal stenting strategy to conventional interventions with long stents. Possibly, the efficacy of spot stenting could be further increased by combining it with additional preparation techniques, such as vessel scoring or debulking.
Electronic supplementary material
The electronic supplementary material (ESM) is available with the online version of the article at https://doi.org/10.1024/0301-1526/a000927
This research could not have been conducted without the help of Denny Herberger at Medical Scientific Affairs B. Braun, Berlin for his logistic support to obtain ethics approvals.
References
1 Nitinol stents in the femoropopliteal artery: a mechanical perspective on material, design, and performance. Ann Biomed Eng. 2018;46(5):684–704.
2 . Limb flexion-induced axial compression and bending in human femoropopliteal artery segments. J Vasc Surg. 2018;67(2):607–13.
3 . Less is more: the “As Less As Reasonably Achievable Stenting” (ALARAS) strategy in the femoropopliteal area. J Cardiovasc Surg (Torino). 2018;59(4):495–503.
4 . Multiple stent delivery system Multi-LOC, a new technology for spot-stenting of the femoropopliteal artery – proof of concept study in a preclinical large animal model. VASA. 2017;46(6):446–51.
5 First clinical experience with the Multi-LOC multiple stent delivery system for focal stenting in long femoro-popliteal lesions. VASA. 2017;46(6):452–61.
6 Focal stenting of complex femoropopliteal lesions with the multi-LOC multiple stent delivery system: 12-month results of the multicenter LOCOMOTIVE study. Cardiovasc Intervent Radiol. 2019;42(2):169–75.
7 Spot stenting versus full coverage stenting after endovascular therapy for femoropopliteal artery lesions. J Vasc Surg. 2019;70(4):1166–76.
8 Comparison of Spot versus long stenting for femoropopliteal artery disease. Ann Vasc Surg. 2019;58:101–7.
9 Outcomes of spot stenting versus long stenting after intentional subintimal approach for long chronic total occlusions of the femoropopliteal artery. JACC Cardiovasc Interv. 2015;8(3):472–80.
10 . Deformation of the femoropopliteal segment: effect of stent length, location, flexibility, and curvature. J Endovasc Ther. 2016;23(6):907–18.
11 . Does diabetes mellitus play a role in restenosis and patency rates following lower extremity peripheral arterial revascularization? A critical overview. Ann Vasc Surg. 2008;22(3):481–91.
12 . Restenosis after endovascular revascularization in peripheral artery disease. VASA. 2015;44(4):257–70.
13 Shared and differential factors influencing restenosis following endovascular therapy between TASC (Trans-Atlantic Inter-Society Consensus) II class A to C and D lesions in the femoropopliteal artery. JACC Cardiovasc Interv. 2014;7(7):792–8.
14 Homocysteine levels, haemostatic risk factors and patency rates after endovascular treatment of the above-knee femoro-popliteal artery. Eur J Vasc Endovasc Surg. 2004;28(4):410–7.
15 . Lesion Revascularisation subsequent to femoropopliteal spot stenting using the multi-LOC stent delivery system. Vivo. 2020;34(1):433–9.
16 . Should we routinely stent the femoropopliteal artery? An interventionalist’s perspective. J Invasive Cardiol. 2015;27(11):E258–61.
17 . Edge stenosis after covered stenting for long superficial femoral artery occlusive disease: Risk Factor analysis and prevention with drug-coated balloon angioplasty. J Endovasc Ther. 2018;25(3):313–9.
18 . Peripheral artery disease: evolving role of exercise, medical therapy, and endovascular options. J Am Coll Cardiol. 2016;67(11):1338–57.
19 1-year results of the ZEPHYR Registry (Zilver PTX for the Femoral Artery and Proximal Popliteal Artery): Predictors of restenosis. JACC Cardiovasc Interv. 2015;8(8):1105–12.
20 Drug coated balloon supported Supera stent versus Supera stent in intermediate and long-segment lesions of the superficial femoral artery: 2-year results of the RAPID Trial. J Cardiovasc Surg (Torino). 2019;60(6):679–85.
21 . Assessment of self-expanding nitinol stent deformation after chronic implantation into the femoropopliteal arteries. EuroIntervention. 2013;9(6):730–7.
22 Optimized drug-coated balloon angioplasty of the superficial femoral and proximal popliteal arteries using the Tack Endovascular System: TOBA III 12-month results. J Vasc Surg. 2020;72(5):1636–1647.e1.



