Mean Heart Rate and Parameters of Heart Rate Variability in Depressive Children and the Effects of Antidepressant Medication
An Exploratory Study
Abstract
Abstract.Introduction: Researchers have repeatedly discovered an association between depression and autonomic cardiac dysregulation in adults. However, corresponding data concerning minors are still rare. Method: For this exploratory, cross-sectional study, we included N = 43 minors (age range 9–17 years). The subjects were depressive subjects with or without antidepressant medication (N = 23) or healthy control children (HC) (N = 20). We assessed several indices of cardiac functioning using long-term electrocardiogram data (mean heart rate, HR, and several parameters of heart rate variability, HRV). We hypothesized that increased HR and reduced HRV are associated with depressive disorders. Furthermore, we assessed the impact of age, sex, and antidepressant medication on HR and HRV. Results: When sex and age were controlled for, HR was significantly increased in depressive minors compared to HC. However, our preliminary data suggest that this might not be the case in medicated patients, and there were no differences between groups regarding HRV parameters. There was no significant correlation in the whole sample between severity of depression and both HR and HRV. In the subsample of patients with depression, antidepressant medication was associated with lower HR and higher indices of HRV. Conclusion: The data indicate an association between depression and altered autonomic cardiac regulation, which can already manifests in minors.
Zusammenfassung.Einleitung: Bei Erwachsenen ist eine Assoziation zwischen Depression und autonomer kardialer Funktion wiederholt beschrieben worden. Daten von Kindern und Jugendlichen liegen kaum vor. Methodik: In einer explorativen Querschnittsuntersuchung wurden N = 43 Kinder und Jugendliche im Alter von 9–17 Jahren untersucht. 23 zeigten eine depressive Störung, die z. T. mit Antidepressiva behandelt wurde. Die gesunde Kontrollgruppe bestand aus 20 Kindern. Mittels Langzeit-EKG wurden die mittlere Herzrate (HR) und verschiedene Parameter der Herzratenvariabilität (HRV) erhoben. Es wurde geprüft, ob eine erhöhte HR und verminderte HRV mit depressiven Störungen assoziiert sind. Zudem wurde der Einfluss von Alter, Geschlecht und antidepressiver Medikation auf HR und HRV bestimmt. Ergebnis: Unter Berücksichtigung des Einflusses von Geschlecht und Alter war die HR in der klinischen Gruppe im Vergleich zur Kontrollgruppe signifikant erhöht. Die explorativen Daten deuten allerdings an, dass dies nicht für medizierte Patienten galt. Keine signifikanten Gruppenunterschiede wurden im Hinblick auf Parameter der HRV gefunden. In der Gesamtgruppe bestand keine signifikante Korrelation zwischen der Ausprägung einer Depression und HR bzw. HRV. In der Subgruppe der depressiven Kinder und Jugendlichen war die Medikation mit verminderter HR und erhöhten HRV-Indizes assoziiert. Schlussfolgerung: Die Daten deuten eine Assoziation zwischen Depression und veränderter autonomer kardialer Funktion bereits bei Kindern und Jugendlichen an.
Introduction
Depression is one of the most frequent and impairing psychiatric disorders worldwide (Murray et al., 2012), with an estimated 1-year prevalence of 4–5 % in mid- to late adolescence (Costello, Egger, & Angold, 2005; Costello, Erkanli, & Angold, 2006). The burden of depression is high since it is associated with substantial present and future morbidity as well as high suicide risk (for a review, see Thapar, Collishaw, Pine, & Thapar, 2012). Depression may also often co-occur with other psychiatric comorbidities such as anxiety or conduct disorders. With respect to comorbid somatic disorders, the association between depression and heart disease is also well documented (for a review, see, e. g., , Kemp et al., 2010; Wulsin & Singal, 2003). Although this fact is descriptively mirrored in many linguistic expressions, such as “dying of a broken heart,” only few scientific studies have focused on this topic (Glassman, 2007), especially concerning children and adolescents (Koenig, Kemp, Beauchaine, Thayer, & Kaess, 2016).
Several mechanisms have been suggested as linking depression to heart functioning. One of the most promising approaches is the association between depression and autonomic cardiac regulation, which can be assessed by measuring heart rate variability (HRV) and which has repeatedly been shown to be altered in adults (Carney, Freedland, Miller, & Jaffe, 2002; Carney, Freedland, & Veith, 2005; Penninx, Milaneschi, Lamers, & Vogelzangs, 2013; Stein et al., 2000). It has been hypothesized that HRV might represent a marker of a person’s emotional adaptability to environmental influences (Appelhans & Luecken, 2006; review by Thayer & Lane, 2009). Data indicate an inverse relationship between reduced vagal activity in depressed adults, as indicated by decreased HRV, and depression severity (Kemp et al., 2010; Yeh et al., 2016). Moreover, investigations in adult samples indicate that the use of antidepressant medication (especially tricyclic antidepressants) might be associated with changes in HRV parameters (O’Regan, Kenny, Cronin, Finucane, & Kearney, 2015), while the data with regard to medication with selective serotonin-reuptake-inhibitors (SSRI) is less consistent (Kemp et al., 2010; Licht, de Geus, van Dyck, & Penninx, 2010).
These findings might have important clinical implications, since autonomic cardiac parameters might assist both diagnostic procedures as well as monitoring of treatment outcome in depressed patients.
Although depressive disorders frequently carry a risk of chronification, beginning in childhood and adolescence (Costello et al., 2002; Rohde, Lewinsohn, Klein, Seeley, & Gau, 2013), data on autonomic cardiac functioning in underage persons with depression are still rare. An exception is the study by rom Gentzler and co-workers (2012) indicating that children with a family history of depression were characterized by an absence of the typical HRV increase during adolescence. Earlier studies focused solely on adolescent girls with depression (Blom, Olsson, Serlachius, Ericson, & Ingvar, 2010; Tonhajzerova et al., 2010; Tonhajzerova et al., 2009). Only one study also included adolescent boys (Byrne et al., 2010). While some found an increased HR and no differences of high-frequency HRV (Byrne et al., 2010), others found decreased indices of HRV (high-frequency, low-frequency, RMSSD, and DNN) (Blom et al., 2010; Tonhajzerova et al., 2010; Tonhajzerova et al., 2009). However, all of these studies – except for Blom et al. (2010) – did not account for treatment effects by antidepressant medication and further assessed electrocardiogram (ECG) data only in unmedicated patients and controls. Recently a meta-analysis (Koenig et al., 2016) indicated reduced vagal functioning (decreased high frequency HRV) in depressive children in contrast to nondepressed controls. However, most of the studies were based on nonclinical samples, and all used short-time ECG.
The current exploratory study measured changes of autonomic cardiac regulation in a group of minors, including children with depressive disorders using long-term 24-hour ECG monitoring. Although short-term ECG is the standard for assessing HRV parameters, the GRAPH guidelines (Quintana, Alvares, & Heathers, 2016) also emphasize that respiratory parameters may modify the relationship between mean heart rate (HR), HRV, and cardiac vagal modulation. Though ambulatory ECG assessments are not superior regarding standardization, we wanted to obtain more ecologically valid data using long-term ECG. We were especially interested in differences of the HR and HRV parameters (DNN, SDANN, RMSSD) in depressive children and adolescents and healthy controls (HC). We expected to find that depressive disorders are associated with increased HR and decreased HRV parameters. We additionally expected that this association is pronounced with increasing depression severity. Furthermore, we wanted to assess the impact of the potential confounding factors age and sex on HR and HRV parameters. In this context, an inverse association between age and HR has repeatedly been shown (e. g., Fleming et al., 2011). Most recently, a meta-analysis also revealed a lower HR in boys than in girls (Koenig, Rash, Campbell, Thayer, & Kaess, 2017). Finally, we explored whether antidepressant medication was associated with changes of HR and HRV parameters.
To our knowledge, this exploratory study is the first observational investigation using long-term ECG to assess the impact of antidepressant medication on parameters of the autonomic cardiac regulation among depressive children and adolescents.
Methods
This exploratory and cross-sectional study was approved by the Ethics Committee of the University of Würzburg in 07/2011 (IRB ID: 108/11).
Sample Recruitment
A total of 33 patients with a suspected depressive disorder and 32 HC participated in the study from January 2012 to February 2015. Written informed consent was obtained from all children/adolescents and their legal guardians.
M. H. and B. v. D. (at the time medicine students working on their doctoral theses) recruited all clinical subjects consecutively from the inpatient and outpatient units of the Center of Mental Health, Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, University Hospital of Würzburg, Germany.
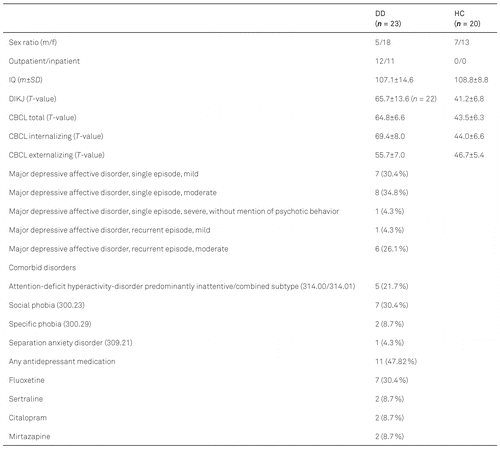
Somatic diseases were assessed in all subjects by means of a survey especially developed for the study, allowing for a systematic anamnestic assessment of frequent somatic disorders, such as diabetes, high blood pressure, allergies, traumatic brain injury, neurological disorders, etc. We screened all subjects for internalizing and externalizing disorders using the CBCL as well as the DIKJ and BDI-II, respectively. In children with a depressive disorder, we additionally performed a further extensive clinical assessment of psychiatric data (see below for details).
In accordance with the inclusion and exclusion criteria for the clinical sample (see Table 1), we excluded one patient because of autism spectrum disorder, six because of medication intake (e. g., methylphenidate, L-thyroxine), and three patients because the diagnosis of a depressive disorder could not be confirmed.

The control group was recruited from a pool of healthy children and adolescents from local schools who already had participated in former studies of the Center of Mental Health. They were selected using an age-matching procedure and consisted of healthy children aged 9 to 17 years without any psychiatric or somatic disorder.
We excluded 12 subjects from the control group because of reported somatic disorders (n = 1) or missing data (n = 6), or because of their values over the cut-off in the screening-questionnaires used (n = 5; details below).
The study sample thus compromised 23 patients with a depressive disorder (11 of whom were taking antidepressant medication) and 20 HC.
Clinical Assessment of Psychometric Data
We administered the Child Behavior Checklist (CBCL, Achenbach, 1991; Döpfner, Schmeck, & Berner, 1994) to screen for internalizing and externalizing symptoms as reported from the parents. Cut-off for the HC was a T-value below 60 in the total score and 60 for the internalizing and externalizing subscores, respectively.
Severity of depressive symptoms was assessed by means of a self-report questionnaire (German version of the Children’s Depression Inventory: “Depressionsinventar für Kinder und Jugendliche,” DIKJ) with good to very good validity and reliability in children aged 9–12 years (for details, see Kovacs, 1985; Stiensmeier-Pelster, Schürmann, & Duda, 2000). The cut-off for the HC was an unstandardized score below 18.
In all subjects of the clinical group, psychiatric disorders were diagnosed by an experienced child and adolescent psychiatrist according to DSM-IV-TR (Saß, 2003; Schneider, Unnewehr, & Margraf, 2009) and confirmed using a comprehensive clinical diagnostic assessment including a semistructured interview (“Diagnostisches Interview bei psychischen Störungen im Kindes- und Jugendalter,” Kinder-DIPS) (Schneider et al., 2009). All participants and their parents were interviewed separately, and the final diagnoses was derived according to a predefined scheme for the assessment of the Kinder-DIPS (for details, see Adornetto, In-Albon, & Schneider, 2008; Schneider et al., 2009). The Kinder-DIPS is characterized by good to very good reliability and validity, with a retest reliability of 98 % for depressive disorders according to the diagnostic interview with the child and of 100 % for the interview with parents (Schneider et al., 2009).
To assess cognitive functioning in all subjects, we applied the Culture Fair Test (CFT20-R), which is a valid and reliable standardized speech-free IQ assessment (Weiß, 2006).
Assessment of ECG
We recorded a long-term 24-hour ECG in all participants during everyday activity using a 3-lead portable ECG-recorder with a sampling rate of 1024 Hz (DMS 300-3A from “MTM multitechmed GmbH”). During ECG recording, all subjects kept a diary and took notes detailing their activities during the day. For the analysis of ECG data, we used the MTM-CardioScan 11 software (DM Software Inc., USA). All ECGs were first manually inspected for the right classification of QRS complexes (usually the central and most visually obvious part of the ECG tracing, corresponding to the depolarization of the right and left ventricles). Artifacts were excluded. Subsequently the following parameters of HRV were determined automatically by the software according to guidelines from the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996):
- •Mean heart rate (HR)
- •Deviation of normal to normal (NN) intervals over a 24-hour period (DNN)
- •Standard deviation of the averages of NN intervals in all 5-min segments of the entire recording (SDANN)
- •Root mean square of successive differences (the square root of the mean of the square of the differences between adjacent NN; RMSSD)
(For details of the measurements of HRV please see McCraty & Shaffer, 2015.)
Statistics
We performed statistical analyses with SPSS-22 (IBM, USA). To test the primary hypotheses on between-group differences in cardiac parameters (HR and the three HRV parameters DNN, SDANN, RMSSD), we calculated one-sided independent t-tests. Between-group differences with respect to the control variables sex and age were tested using Fisher’s exact test (sex by group) and independent t-test (age by group). We examined associations between the control variables sex and age with the cardiac parameters using point-biserial and Pearson’s coefficients. To test the primary hypotheses controlling for sex and age, we performed ANCOVAs (cardiac parameters by group with sex and age as covariates). To examine the association between depression severity and cardiac parameters, we correlated T-values of the DIKJ sum score with cardiac parameters using Spearman’s coefficient rho. The effect of medication was assessed in the subsample of depressive patients correlating medication intake as dichotomous variable with the cardiac parameters (point-biserial correlation).
Results
Sample Characteristics
We examined N = 23 subjects with depressive disorder (DD) (11 with antidepressant medication) (mean-age 13.87 ± 2.2 years), and N = 20 healthy controls (mean-age 14.25 ± 1.94 years). We found no significant differences between the two groups with respect to age or sex (age: t = 0.59, df = 41, p = .56; sex: Fisher exact p = .497). Table 2 presents the demographic characteristics of the sample.
Cardiac Parameters
In accordance with our hypotheses, depressive minors had a significantly higher mean HR than controls. HRV parameters were descriptively smaller in depressive patients, but these differences failed to be statistically significant (see Table 3).
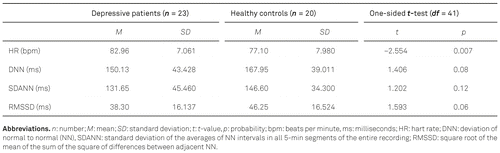
Against our hypothesis there was neither a significant positive association between severity of depression (as measured by the DIKJ T-values) and HR nor were there significant negative correlations between severity of depression and HRV parameters (n = 42; HR: Rho = 0.145, p = .180; DNN: Rho = –0.159; p = .158; SDANN: Rho = –0.154, p = 0.165; RMSSD: Rho = –0.165, p = 0.148). However, the direction of these correlations was in line with our assumptions.
With respect to potential confounding factors, as expected our correlation analyses revealed a negative association between age and HR (n = 43, r = –0.35, one-sided p = .01) as well as that boys were characterized by a significantly reduced HR (n = 43, rpbi = 0.37, one-sided p < .01). No significant correlations were found between age or sex and HRV parameters.
Our results on differences between depressive patients and controls in cardiac parameters presented in Table 3 were not adjusted for age and sex. The two-group ANCOVA for HR resulted in a significant model (N = 43, R2 = 0.33, sum of squares: 827.3, df = 3, F = 6.28, p < .001) with significant effects for the factors age, sex and group (age: F = 4.90, sex: F = 4.93, group: F = 5.06; all p-values < .05). Thus, differences between groups were still present when controlling for age and sex. All ANCOVAs for HRV parameters were nonsignificant.
Furthermore, we analyzed the association between an antidepressant medication and cardiac parameters within the clinical sample and found a significant reduced HR in depressed minors with medication compared to those without medication (n = 23, rpbi = –0.46, p = .02). HRV was slightly higher but not statistically significant in patients with medication (n = 23; DNN: rpbi = 0.34, p = .12; SDANN: rpbi = 0.32, p = .07; RMSSD: rpbi = 0.29, p = .18; all reported p-values are two-sided).
Discussion
The present exploratory study investigated whether depressive disorders are associated with an increased HR and reduced parameters of HRV in children and adolescents. While a group difference between depressed minors and HC has already been reported in adult samples, a limited number of studies in minors reported contradicting results (e. g., Blom et al., 2010; Byrne et al., 2010). Furthermore, those studies focused on highly selected samples, i. e., only adolescent girls (Blom et al., 2010; Tonhajzerova et al., 2010; Tonhajzerova et al., 2009), or could not rule out an influence of a current antidepressant medication on observed differences of the HRV (Blom et al., 2010). In our study, we included boys and girls and used ambulatory 24-hour ECG to examine HR and HRV.
As expected, we found that depressive children and adolescents had a significantly higher HR compared to the HC, and that this effect continued when results were controlled for the impact of age and sex (see below). Hence, the results of our exploratory study agree with former studies concerning depressive adolescents (Byrne et al., 2010) and girls with depression (Blom et al., 2010; Tonhajzerova et al., 2009; Tonhajzerova et al., 2010) and expand this finding also for boys and children. Our results emphasize that young depressed minors who are physically healthy already differ significantly from age-matched HC with respect to HR. However, we were not able to exclude the effects of antidepressant medication. Furthermore, we did not find the expected reduction of HRV in depressed minors described in former studies (e. g., Blom et al., 2010). Note that we recorded long-term ECGs, while most other studies on this topic used short-term recordings. Although Kemp et al. (2010) found significant differences of HRV between depressed adult patients and HC in both long- and short-term recordings, interpreting HRV in long- and short-term recordings is a different task: Frequency domain HRV parameters used in short-term recordings allow a precise examination of the different components of cardiac autonomic function, while time-domain HRV parameters used in long-term recordings give quantitative information about the overall sympatho-vagal balance (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996).
In contrast to our hypothesis, we did not find a positive correlation between the severity of depression and HR, nor did we find an inverse association with HRV parameters as described previously in adult samples (Kemp et al., 2010; Yeh et al., 2016). One could argue that, from a clinical point of view, often only low correlations occur especially in children and adolescents between self-ratings of depressive symptoms and the clinical impression of the individual’s severity of depression by experts. To some extent this may explain our negative finding. Again, recall that we present data from an exploratory study with a small sample size.
As an additional finding, our correlational analyses revealed the expected inverse association between age and HR, which has repeatedly been described in the literature (e. g., Fleming et al., 2011), as well as the finding that boys had a significant lower HR than girls within the whole sample (meta-analysis by Koenig et al., 2017).
Finally, within the clinical sample we found that antidepressant medication was associated with a reduced HR, whereas the HRV parameters were not significantly increased. This contrasts with findings from a large longitudinal study from O’Regan et al. (2015), who found that adults with depressive disorders taking antidepressants had lower measures of HRV and increased HR relative to controls. Hence, one could argue that the preliminary results of our exploratory study indicate that SSRI seem to be associated with HR and HRV changes opposite to adults taking antidepressant medication. However, conclusions should be drawn with care since our sample was quite small and two children in the clinical group were not medicated with a SSRI but mirtazapine. Recent studies in adult samples indicate that antidepressant medications seem to differ significantly with respect to their impact on measures of HRV (O’Regan et al., 2015). Moreover, in contrast to our sample, the sample in the O’Regan study was composed of older individuals, who metabolize and respond to antidepressants differently than children and adolescents.
Limitations and Strengths
The present exploratory study contains several limitations that deserve to be mentioned. First, the sample size was quite small. We may have failed to uncover some associations because of reduced statistical power. It is a methodological weakness of our study that we did not perform an a-priori sample size estimation. Second, some of the minors had somatic or psychiatric comorbidities that might be independently associated with abnormalities in autonomic cardiac regulation (see, e. g., Chalmers, Quintana, Abbott, & Kemp, 2014; Ebinger, Kruse, Just, & Rating, 2006; Rash & Aguirre-Camacho, 2012). Although we controlled our data with respect to the potential confounding factors age and sex, we did not adjust our data regarding potential confounders like comorbid anxiety disorders or body mass index. On the other hand, this accurately reflects clinical reality since populations with depressive disorders typically suffer from comorbid conditions (Penninx et al., 2013). At least we defined the diagnosis of an eating disorder as an exclusion criterion.
We used long-term ECG for HR and HRV analyses, which is not common (Quintana et al., 2016). Although we did this in order to increase the standardization and the chance of habituation in the ECG assessment in depressed children, it makes our results difficult to interpret and reduces comparability with prior studies. Unfortunately, our ECG software does not provide hourly HRV values, so that we could not do cosinor analysis, which would have provided more detailed information about circadian variation of HRV (see, e. g., Buchhorn et al., 2018). Moreover, the absence of frequency domain measurements is a further limitation of our study. Further, we should emphasize that multiple analyses were made with no correction for alpha levels, which increases the likelihood of false positive results.
Conclusion and Outlook
In this exploratory study of a small number of depressed children and adolescents of mixed sex we found increased HR in depressed minors compared to HC and thereby replicated earlier studies in adult samples. We also found a normalization of HR associated with antidepressant medication. In light of our finding of high risk of chronic courses of depressive disorders, especially early manifestations, we emphasize the need for further investigations into the associations between depression and antidepressant medication. Longitudinal studies in children are urgently recommended.
Future studies among minors should also analyze frequency domain HRV in short-term recordings for better data interpretation. When analyzing long-term ECGs, one should use cosinor analysis (see, e. g., Buchhorn et al., 2018) to get more detailed information about circadian patterns of HRV. Since frequent confounding variables, such as demographic factors, fitness, weight, and psychiatric and somatic comorbidities, influence autonomic cardiac regulation, larger sample sizes are needed in studies on the association between depressive disorders and heart functioning to control for those confounders. However, since cardiac autonomic parameters may potentially support therapeutic and diagnostic issues in depressed minors, larger studies in minors are urgently needed –and also seem feasible – since data assessment is noninvasive and generally well tolerated.
Literature
(1991). Manual for the Child Behavior Checklist/4–18 and 1991 profile. Burlington VT: Department of Psychiatry, University of Vermont.
(2008). Diagnostik im Kindes- und Jugendalter anhand strukturierter Interviews: Anwendung und Durchführung des Kinder-DIPS. Klinische Diagnostik und Evaluation, 1, 363–377.
(2006). Heart rate variability as an index of regulated emotional responding. Review of General Psychology, 10, 229–240.
(2010). Heart rate variability (HRV) in adolescent females with anxiety disorders and major depressive disorder. Acta Paediatrica, 99, 604–611.
(2018). A case series on the potential effect of omega-3-fatty acid supplementation on 24-h heart rate variability and its circadian variation in children with attention deficit (hyperactivity) disorder. Attention Deficit and Hyperactivity Disorders. 10, 135–139.
(2010). Autonomic cardiac control in depressed adolescents. Depression and Anxiety, 27, 1050–1056.
(2002). Depression as a risk factor for cardiac mortality and morbidity: A review of potential mechanisms. Journal of Psychosomatic Research, 53, 897–902.
(2005). Depression, the autonomic nervous system, and coronary heart disease. Psychosomatic Medicine, 67 Suppl 1, S29–S33.
(2014). Anxiety disorders are associated with reduced heart rate variability: A meta-analysis. Frontiers in Psychiatry, 5, 80.
(2005). 10-year research update review: The epidemiology of child and adolescent psychiatric disorders: I. Methods and public health burden. Journal of the American Academy of Child and Adolescent Psychiatry, 44, 972–986.
(2006). Is there an epidemic of child or adolescent depression? Journal of Child Psychology and Psychiatry, 47, 1263–1271.
(2002). Development and natural history of mood disorders. Biological Psychiatry, 52, 529–542.
(1994). Handbuch: Elternfragebogen über das Verhalten von Kindern und Jugendlichen. Forschungsergebnisse zur deutschen Fassung der Child Behavior Checklist (CBCL) (1. Aufl.). Köln: KJFD, Arbeitsgruppe Kinder-, Jugend- und Familiendiagnostik.
(2006). Cardiorespiratory regulation in migraine: Results in children and adolescents and review of the literature. Cephalalgia, 26, 295–309.
(2011). Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: A systematic review of observational studies. Lancet, 377, 1011–1018.
(2012). Atypical development of resting respiratory sinus arrhythmia in children at high risk for depression. Developmental Psychobiology, 54, 556–567.
(2007). Depression and cardiovascular comorbidity. Dialogues in Clinical Neuroscience, 9, 9–17.
(2010). Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biological Psychiatry 67, 1067–1074.
(2016). Depression and resting state heart rate variability in children and adolescents: A systematic review and meta-analysis. Clinical Psychological Review. 64, 136–150.
(2017). A meta-analysis on sex differences in resting-state vagal activity in children and adolescents. Frontiers in Physiology, 8, 582.
(1985). The Children’s Depression Inventory (CDI). Psychopharmacology Bulletin, 21, 995–999.
(2010). Longitudinal evidence for unfavorable effects of antidepressants on heart rate variability. Biological Psychiatry, 68, 861–868.
(2015). Heart rate variability: New perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Global Advances in Health and Medicine, 4, 46–61.
(2012). Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet, 380, 2197–2223.
(2015). Antidepressants strongly influence the relationship between depression and heart rate variability: Findings from The Irish Longitudinal Study on Ageing (TILDA). Psychological Medicine, 45, 623–636.
(2013). Understanding the somatic consequences of depression: Biological mechanisms and the role of depression symptom profile. BMC Medicine, 11, 129.
(2016). Guidelines for reporting articles on psychiatry and heart rate variability (GRAPH): Recommendations to advance research communication. Translational Psychiatry, 10, 6.
(2012). Attention-deficit hyperactivity disorder and cardiac vagal control: A systematic review. Attention Deficit and Hyperactivity Disorders, 4, 167–177.
(2013). Key characteristics of major depressive disorder occurring in childhood, adolescence, emerging adulthood, adulthood. Clinical Psychological Science, 1.
(2003). Diagnostisches und statistisches Manual psychischer Störungen – Textrevision – DSM-IV-TR ; übersetzt nach der Textrevision der vierten Auflage des Diagnostic and Statistical Manual of Mental Disorders der American Psychiatric Association. Göttingen: Hogrefe.
(2009). Kinder-DIPS – Diagnostisches Interview bei psychischen Störungen im Kindes- und Jugendalter (2., aktualisierte und erw. Aufl.). Heidelberg: Springer-Verlag.
[please supply last author??] (2000). Severe depression is associated with markedly reduced heart rate variability in patients with stable coronary heart disease. Journal of Psychosomatic Research, 48, 493–500.
(2000). Depressions-Inventar für Kinder und Jugendliche – DIKJ (2. überarb. und neunormierte Aufl.). Göttingen: Hogrefe.
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology . (1996). Heart rate variability. Standards of measurement, physiological interpretation, and clinical use . European Heart Journal, 17, 354–381.(2012). Depression in adolescence. Lancet, 379, 1056–1067.
(2009). Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews, 33, 81–88.
(2010). Cardiac autonomic regulation is impaired in girls with major depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry and Clinical Neurosciences, 34, 613–618.
(2009). Respiratory sinus arrhythmia is reduced in adolescent major depressive disorder. European Journal of Medical Research, 14 Suppl 4, 280–283.
(2006). Grundintelligenztest Skala 2, CFT 20-R (rev. ed.). Göttingen: Hogrefe.
(2003). Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosomatic Medicine, 65, 201–210.
(2016). Heart rate variability in major depressive disorder and after antidepressant treatment with agomelatine and paroxetine: Findings from the Taiwan Study of Depression and Anxiety (TAISDA). Progress in Neuropsychopharmacology and Biological Psychiatry, 64, 60–67.



