Psychosocial Predictors of Sleep Quality in Residents of Nursing Homes
Abstract
Abstract. This article examines the cross-sectional and long-term prediction of sleep quality (SQ) of 167 older nursing-home residents (80% females, 69–100 years), who participated in the study in 2008 and 2016. SQ was assessed in 2016 by the Pittsburgh Sleep Quality Index (PSQI); Total PSQI was found to be greater than 5 in 71% of participants. The domains of Subjective SQ and Daytime Functioning were relatively good, while Sleep Efficiency was most impaired. The observed set of predictors significantly explained 7–13% of PSQI variance cross-sectionally and 12–18% in the long-term effects analyses. The structure of predictors differed across SQ domains, in both the cross-sectional and the long-term effects analyses, and between the two, indicating the important impact of changes in psychophysiological functioning for current SQ of older adults.
Normal human aging is associated with observable changes in sleep characteristics that take place continuously over the lifespan. Studies have shown that sleep in older adults is characterized by an earlier circadian phase, a shortening of overall sleep duration, a decrease in deep slow-wave sleep stages and REM sleep, and an increase in lighter sleep stages (Bloom et al., 2009; Ohayon, Carskadon, Guilleminault, & Vitiello, 2004). The meta-analysis by Ohayon et al. (2004) demonstrated that most changes in sleep architecture took place up to the age of 60, after which the only sleep parameter that significantly deteriorated in healthy older adults was sleep efficiency. In normally aging older adults, the developmental changes in the sleep process do not necessarily result in sleep problems and impairments (Bloom et al., 2009; Miner & Kryger, 2017). However, over 83% of older adults in fact experience some health conditions (Foley, Ancoli-Israel, Britz, & Walsh, 2004). According to other authors, around 90% of adults older than 65 treat their health conditions pharmacologically (Ancoli-Israel, 2011; Li, Vitiello, & Gooneratne, 2018). Both health conditions and the medication taken for them can be disruptive to sleep-wake behavior. For example, nocturia, chronic pain, cardiovascular diseases, respiratory diseases, limited mobility, depression, and dementia can by themselves, or via the medications used to treat them, disrupt sleep by shortening or prolonging sleep onset and offset, and/or by fragmenting sleep with multiple nighttime awakenings. Insufficient, poor-quality sleep may additionally contribute to a decline in daily functioning and be related to different adverse health effects including higher mortality risk (Cappuccio, D’Elia, Strazzullo, & Miller, 2010; Martin et al., 2011).
Sleep quality (SQ) is a complex construct that is not easily defined (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). It sometimes consists of only one and at other times a combination of several quantitative and/or qualitative measures of the sleep process. It can comprise sleep latency (the time needed to fall asleep), frequency and duration of nighttime awakenings, frequency of nightmares or bad dreams, sleep duration, regularity of sleep schedule, use of different aids to promote sleep or wakefulness, sleep efficiency (the ratio between time asleep and time in bed), the subjectively estimated quality of one’s sleep, and daytime functioning measures (sleepiness, drowsiness, alertness, restedness).
In the US National Sleep Foundation Sleep and Aging Poll of 2003, which included community-dwelling older adults between 65–84 years, 33% respondents reported fair to poor SQ, with 57% reporting having experienced at least some kind of sleep-related problem in the previous year (Foley et al., 2004). Spira et al. (2012) reported clinically impaired SQ as measured by the Pittsburgh Sleep Quality Index (PSQ) in 39–45% of community-dwelling older males, both black and white. Beaudreau et al. (2012) reported impaired SQ by the same criterion in 51–59% of community-dwelling older females. In a very large sample of Chinese adults, predominantly community dwelling, between 65–100 years, poor SQ was self-reported in 35% of the respondents (Gu, Sautter, Pipkin, & Zeng, 2010). St George, Delbaere, Williams, and Lord (2009) found poor self-rated SQ over the previous month in 15% of adults 65–95 years living in various housing conditions, with a much higher percentage (37%) also reporting restless sleep.
As Neikrug and Ancoli-Israel (2010) summarized, around 50% of older adults report poor SQ, predominantly associated with impaired physical and/or mental health. Deterioration in health and lack of instrumental social support sometimes lead to the necessity of nursing-home-type assistance in daily living activities. In addition to the personal biological and psychosocial factors, living in an institution adds additional environmental factors that impact the already vulnerable sleep-wake process of older people (Neikrug & Ancoli-Israel, 2010). Such factors, for example, include less exposure to daylight, more light and noise at night, the habits and needs of roommate(s), the timing of meals and schedules of other organizational routines like cleaning and maintenance. Azri, Dahlan, Masuri, and Isa (2016) found that 50% of adults 60–97 years residing in institutions for the elderly in Malaysia reported significantly impaired SQ as measured by the PSQI. The study by Valenza et al. (2013) showed that SQ was significantly impaired in over 72% of nursing-home residents, while Martin, Fiorentino, Jouldjian, Josephson and Alessi (2010) reported PSQI indicating poor SQ in 65% older adults in assisted-living facilities.
The results of the cross-sectional studies to date showed that factors like better socioeconomic status, better physical and mental health, better functional ability, more physical activity, less medication use, availability of healthcare, living with a spouse, and having available other sources of social support significantly contributed to better SQ of older adults (Dregan & Armstrong, 2011; Gindin et al., 2014; Gu et al., 2010; St George et al., 2009; Ohayon, Zulley, Guilleminault, Smirne, & Priest, 2001; van de Straat & Bracke, 2015).
The results from research studies on sleep in older adults stem predominantly from the American and Asian countries, while data for European countries are fewer, especially pertaining to normal sleep characteristics (Košćec Bjelajac, Holzinger, Despot Lučanin, Delale, & Lučanin, submitted). To the best of our knowledge, only one published study has analyzed sleep patterns and quality in older adults in Croatia (Štefan, Vučetić, Vrgoč, & Sporiš, 2018). Furthermore, the predictors of SQ in older adults in other countries predominantly examined them cross-sectionally. Therefore, the results of our study should contribute to broadening the knowledge on SQ in a group of older adults living in nursing homes in yet another sociocultural context. This study first examines SQ in older adults residing in nursing homes in Zagreb and second explores how a set of biological and psychosocial factors contributed to the cross-sectional prediction of SQ as well as the effects of changes in these factors on the prediction of current SQ over an 8-year period.
Method
Participants
The participants were 167 residents from 10 city-owned nursing homes in Zagreb, Croatia (80.2% females, 69–100 years, M = 84.5, SD = 6.13), who participated in the study at three measurement points – in 2008 (N = 561), 2010 (N = 410), and 2016 (N = 167). All participants were ambulatory and without a diagnosis of dementia, which were the eligibility criteria.
For the selection of participants at baseline, social workers or head nurses in each nursing home were instructed to approach approximately 10–15 or more residents (depending on the size of the nursing home), approximately sex balanced (if possible), from four age groups: 65–70, 70–75, 75–80, 80 and older. The residents were informed of the research procedure and invited to participate and to agree with the predetermined criteria: that they were ambulatory and without diagnosis of dementia. The residents’ interest in participation was huge, even higher than planned, also because the baseline measurement included a medical examination (not presented here).
At the last measurement point, 366 (65%) of the participants from the baseline measurement had died, and 28 (5%) were unwilling, had relocated, or were incapable of participating. Analyses of variance were employed to test the differences in observed variables at baseline and at second‐time measurement between those participants who took part at all three measurement points and those that did not because of having deceased. Statistically significant differences were found between the surviving and the deceased participants in all observed variables (at p < .01), except for the self‐perceived health variable at baseline measurement (although borderline at p = .056). The means of the variables showed significantly better baseline functioning and younger age of the surviving participants compared to the deceased ones, a finding common in longitudinal studies.
Procedure
This study represents a continuation of a longitudinal project on the biopsychosocial predictors of survival and health in old age which began in 2008. The initial sample consisted of the nursing-home residents who had agreed to participate at baseline in 2008 and two follow‐ups with the surviving participants in 2010 and in 2016. For the purposes of the present research report, only data from the baseline (in 2008) and the third measurement point (in 2016) were analyzed and presented. This occurred, first, because of the greater expected time effects between the two furthermost measurement points and, second, because of unequal distances between the measurement points – 2 years between baseline and the second measurement and 6 years between second and third measurement points. The data were individually collected at the nursing homes by trained interviewers, in the form of structured interviews. The study was conducted in accordance with the Declaration of Helsinki, Code of Ethics of the University of Zagreb, and the Code of Ethics of the Croatian Psychological Association. Participation was voluntary, and all participants provided signed consent at each measurement point.
Instruments
The questionnaire on sociodemographic characteristics was administered at all three measurement points. Besides other general information, it comprised questions concerning age, sex, marital status, and chronic illnesses.
Self-perceived health was assessed by the participants’ rating their general health (“How would you rate your health?”), on a response scale from 1 = very bad to 5 = excellent, and by comparing their subjective health to that of their age peers (“Compared to your peers, how would you rate your health?”) on a three-point scale from 1 = worse to 3 = better. The scores on the two questions were summed, resulting in a total score ranging from 2–8, with a higher score indicating better self-perceived health. The Cronbach’s alpha internal consistency coefficient of the Perceived Health Scale in the present study was α = .56.
Functional ability was assessed by Activities of Daily Living Scale (Defilipis & Havelka, 1984; Despot Lučanin, 2003), comprising 14 items (e.g., “Can you lay on and take off your clothes on your own?” or “Can you walk for at least 400 meters?”) measuring the degree of independence in performing daily activities (personal care, walking inside and outside of home, basic domestic chores), on a four-point scale from 1 = totally dependent on other people’s help, to 4 = totally independent. Total scores range between 14–56, with a higher score indicating better functioning. The Cronbach’s alpha coefficient of this scale for older persons in different research in Croatia was .96 (e.g., Despot Lucanin, Lucanin, & Havelka, 1997) and in the present study .91.
Cognitive function was assessed by the Cognitive Function Scale of the Clifton Assessment Procedures for the Elderly (CAPE, by Pattie & Gilleard, 1996). The scale was first translated into Croatian, using methods of parallel-blind translation, backtranslation, and consensus. The CAPE consists of 12 information/orientation items (e.g., “What is your birth date?” or “What is the Prime Minister’s name?”), scored with 1 point each, of three mental ability tasks scored 0‐3 points (counting from 1 to 20, reciting the alphabet, word-list reading), and of a name signing task scored 0‐2 points. Thus, total scores range from 0 to 23, with higher scores indicating better cognitive functioning. Scores less than 15 indicate a mild cognitive decline and less than 8 a considerable cognitive decline. The test-retest reliability indices for total score in this study ranged from .42 (2008–2016) to .54 (2008–2010).
The Social Participation Scale consisted of 5 items (e.g., “Do you participate in the following activities: cultural, like theatre plays or concerts?”) measuring the frequency of participation in different social activities (e.g., cultural, religious), on a 3-point scale from 1 = never to 3 = often. Scores range from 5 to 15, with a higher score indicating more frequent social participation. The internal consistency coefficient of this scale for older persons in different researches in Croatia was α = .61 (Despot Lučanin, 2003) and in the present study α = .60.
The Depression Scale for Older Adults (Bowling, 1995; Despot Lučanin, 2003) was administered in 2008 and 2010. The scale includes 20 items measuring the frequency of different depressive symptoms (e.g., “I feel like crying” or “I get tired for no reason”), on a 4-point scale from 1 = almost never to 4 = almost always, but not assessing clinical depression. The results can range from 20 to 80, with a higher score indicating more depressive symptoms. The internal consistency coefficient in the present study was α = .78 at baseline and α = .80 at the second point of measurement in 2010 (Lučanin, Despot Lučanin, Košćec Bjelajac, & Delale, 2017).
Because the measurement in 2016 was the last planned study wave, in 2016 we substituted the Depression Scale with the Life Satisfaction Scale (Defilipis & Havelka, 1984), since we were more interested in the outcome status of these participants after their 8-year follow-up. The Life Satisfaction Scale comprises 8 items measuring the degree of satisfaction with different aspects of life (e.g., “Do you feel lonely?” or “Are you satisfied with your current financial situation?”), on a 3-point scale from 1 = often/mostly no, to 3 = never/mostly yes. Scores can range from 8–24, with a higher score indicating better life satisfaction. The internal consistency of this scale for older persons in different research in Croatia was α = .80 (Despot Lučanin, 2003) and in present study α = .73.
SQ during the previous month was measured by the Pittsburgh Sleep Quality Index (PSQI – Buysse et al., 1989), albeit only in the last study wave, as the outcome variable (Koscec, Despot Lucanin, Delale, & Lucanin, 2016). The PSQI comprises four questions about sleep timing and duration over the previous month, 13 questions on different aspects of sleep rated on a 4-point scale: 0 = not during the past month, 1 = less than a week, 2 = once or twice a week, 3 = three or more times a week (e.g., “During the past month, how often have you had trouble sleeping because you had to get up to use the bathroom? “), and one question concerning overall SQ during the previous month rated on a 5-point scale from 0 = very good to 3 = very bad. The total score can range from 0–21, with higher scores indicating poorer SQ. A total score greater than 5 was identified as a cut-off point discriminating poor from good sleepers (Buysse et al., 1989). The results on the PSQI can be presented through seven domains: subjective SQ, Sleep Latency, Sleep Duration, Sleep Efficiency, Sleep Disturbances, Use of Sleep Medication, and Daytime Disturbances. The scores in each domain can range from 0–3, with higher scores again indicating poorer SQ in each domain.
The scale was translated into Croatian by five psychologists fluent in English, three of whom were experts in the field of sleep research, using methods of parallel-blind translation, backtranslation, and consensus. Independent translations of the PSQI were highly similar in semantic, idiomatic, and conceptual sense, and any slight discrepancies were resolved by the consensus of all translators (Tsang, Royse, & Terkawi, 2017). The Croatian translation in our sample of 167 nursing-home residents demonstrated relatively low internal consistency (α = .67) compared to the internal consistency obtained in an original study by Buysse et al. (1989 α = .83), and to some other studies (e.g., Carpenter & Andrykowski, 1998) α = .80; Hinz et al., 2017 α = .75). However, the meta-analysis by Mollayeva et al. (2016) reported PSQI Cronbach’s alpha coefficients below .70 in several studies, one of which involved community-dwelling older men (Spira et al., 2012). Beaudreau et al. (2012) reported an internal consistency coefficient of .72 in a sample of community-dwelling older women.
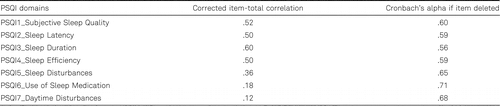
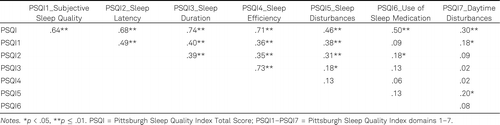
Table 1 presents the data from the internal consistency analysis. The domains Use of Sleep Medication and Daytime Disturbances had very low correlation with the PSQI global score, indicating their poor reliability. Table 2 shows the correlations between all variants of the PSQI scores (between domains and domain total). The Use of Sleep Medication and Daytime Disturbances domains had overall the weakest association with other domains of the PSQI. In addition, Daytime Disturbances had weak correlation with the PSQI Total Score (.30). Removing these two domains (use of Sleep Medication and Daytime Disturbances) would result in somewhat greater internal consistency index of α = .75. Spira et al. (2012) and Beaudreau et al. (2012) also reported a low reliability of these two domains of the PSQI in older adults but decided to keep them in the analyses. In order to have a comparable PSQI Total Score ranging from 0–21 and a proposed cut-off point of 5, we also decided to keep these two domains in our analyses.
Results
Characteristics of Participants
In 2016, 63.7% of participants were widowed, 15.5% had never been married, 12.5% were still married, 6.5% were divorced, and 1.2% declared other marital status. Compared to the data in 2008, we observed that 21% of participants had changed their partnership status in various directions.
Regarding educational level, 47% of the participants had low education level, 40% had graduated from high school, and 12% had a high education level. The great majority of participants reported having some chronic illness or physical impairment (92.2%).
Description of Measures
Table 3 shows the descriptive results for the examined psychosocial measures. The means of the variables estimated at two measurement points were compared by the paired samples t-tests.
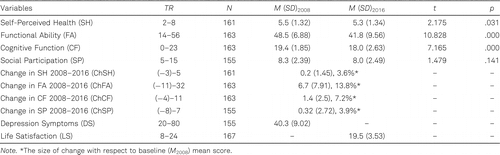
As Table 3 shows, there was a significant decrease in most variables in 2016 compared to 2008 – in functional ability and cognitive function (p < .001), and in self-perceived health (p < .05), while no statistically significant differences were observed in social participation between two measurement points (p > .05). The change in observed variables from 2008 to 2016 measurement points was calculated by subtracting the 2016 score from the 2008 score (score change = 2008–2016). In the case of lower score in 2016, denoting deterioration, the score change was positive, while with a higher score in 2016, denoting improvement, the score change was negative. The variables were Change in Self-Perceived Health 2008–2016, Change in Functional Ability 2008–2016, Change in Cognitive Function 2008–2016, and Change in Social Participation 2008–2016. Table 3 shows the score change range, the mean score change, and the size of change with respect to baseline (2008) mean score for the observed variables.
The descriptive data for the PSQI scale and the seven domains are presented in Table 4. The most impaired was Sleep Efficiency followed by Sleep Latency, Use of Sleep Medications, and Sleep Disturbances, while the best rated aspects of SQ were Daytime Disturbances and Subjective Sleep Quality. Total score on PSQI greater than 5, indicating significantly impaired SQ, was found in 71% of participants. 54% of participants reported taking some medication for sleep in the previous month, 51% of whom reported taking benzodiazepines, 34% did not know which medication they had taken, did not give answer, or reported taking medication unrelated to sleep; 1% took some kind of herbal remedy, and 11% reported taking nonbenzodiazepines.
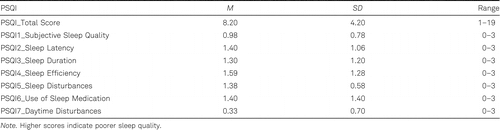
The scores distributions of the PSQI domains 1–7 were significantly positively skewed, confirmed by the Kolmogorov‐Smirnov test (all at p < .01). Even though some impairment of SQ in older adults may be expected, there were still relatively more participants reporting fewer problems with various aspects of their sleep. The distributions of almost all predictor variables were significantly negatively skewed, except for age. Normalization of distributions was not performed since there were no extreme skewness cases, and it furthermore would have interfered with further interpretation of the associations among variables (Tabachnick & Fidell, 2007).
Prediction of Sleep Quality
Table 5 presents the bivariate correlations between predictor variables at two measurement points.
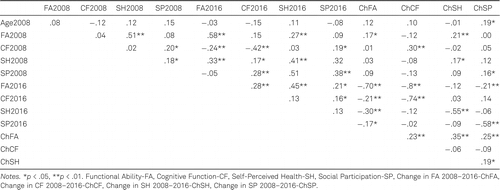
The intercorrelations between predictors of SQ assessed at two timepoints were predominantly low to moderate, ranging from −.16 (p < .05, between Cognitive Function in 2016 and Social Participation in 2016), to .45 (p < .01, between Functional Ability and Self-Perceived Health in 2016). The highest intercorrelations were between each predictor variable measured in 2016 and the change in the variables’ scores from 2008 to 2016, i.e., between Functional Ability in 2016 and Change in Functional Ability 2008–2016 (−.74, p < .001), between Cognitive Functioning in 2016 and Change in Cognitive Functioning 2008–2016 (−.70, p < .001), between Self-Perceived Health in 2016 and Change in Self-Perceived Health 2008–2016 (−.55, p < .001), and between Social Participation in 2016 and Change in Social Participation 2008–2016 (−.58, p < .001), which can be regarded as a method-effect, meaning that a negative correlation coefficient shows that a high score value in 2016 was related to a small difference in scores, or a small change, between 2008 and 2016 measurements.
Table 6 presents the bivariate correlations between PSQI scores and predictors assessed in 2016. They ranged between −.16 (p < 0.05, between Functional Ability and Use of Sleep Medication) to −.32 (p < 0.01, between Self-Perceived Health and Subjective Sleep Quality). Bivariate correlations between PSQI scores and predictors assessed in 2008, as well as the change between 2008 and 2016 ranged between −.16 (p < 0.05, between Cognitive Functioning in 2008 and Daytime Disturbances in 2016) to −.27 (p < 0.01, between Self-Perceived Health in 2008 and Subjective Sleep Quality in 2016) and are presented in Table 7.
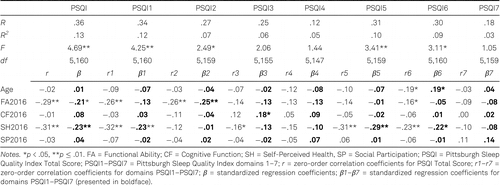
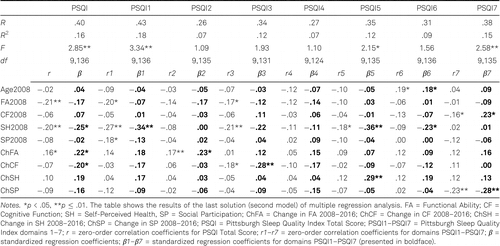
Prior to performing the regression analyses, bivariate correlations among the predictor variables were examined for multicollinearity. As Table 5 shows, no predictors correlated very highly (above .80). Tolerance statistics were all greater than .2, and variance inflation factors (VIFs) were all less than 5, within ranges indicating no multicollinearity problems (Bowerman & O’Connell, 1990).
Multiple regression analyses were performed in order to examine whether SQ in nursing-home residents could be predicted cross-sectionally (Table 6) and over the long-term, including 8-year period changes (Table 7), by the set of biopsychosocial characteristics: chronological age, independence in performing daily tasks, cognitive functioning, self-perceived health, and participation in social activities. The dependent variables were the PSQI Total Score and the scores in each of seven PSQI domains.
Cross-sectionally, the selected set of variables significantly predicted the PSQI Total Score, Subjective Sleep Quality, Sleep Latency, Sleep Disturbances and Use of Sleep Medication, explaining between 7–13% of variance in a particular criterion. As can be seen from Table 6, Self-Perceived Health was the strongest individual cross-sectional predictor of SQ, with better Self-Perceived Health predicting lower total PSQI score (i.e., overall better SQ), and lower scores in three PSQI domains (i.e., better Subjective Sleep Quality, less Sleep Disturbances, and less Use of Sleep Medication). Functional Ability was also a significant cross-sectional predictor of SQ, with better Functional Ability predicting lower total PSQI score (overall better SQ), and lower Sleep Latency score (i.e., shorter Sleep Latency). In addition, older Age was predictive of more frequent Use of Sleep Medication.
Regarding the effects of 8-year period changes in the set of selected variables on the prediction of SQ in 2016, we observed that four significant regression models predicted the PSQI Total Score, Subjective Sleep Quality, Sleep Disturbances and Daytime Disturbances. The amount of explained variance in particular domain varied between 12–18%. Regarding the individual predictors, SQ of nursing-home residents in 2016 could be predicted by Self-Perceived Health in 2008 and Cognitive Functioning in 2008. Better Self-Perceived Health in 2008 predicted better overall SQ, shorter Sleep Latency and fewer Sleep Disturbances (i.e., lower scores) in 2016, whereas better Cognitive Functioning in 2008 predicted more Daytime Disturbances in 2016. Also, SQ of nursing-home residents in 2016 was significantly predicted by the changes in Functional Ability, Cognitive Functioning, Self-Perceived Health, and Social Participation between 2008 and 2016. Bigger change in Functional Ability (i.e., bigger deterioration) from 2008 to 2016 predicted worse overall SQ (i.e., higher score) in 2016, and bigger deterioration in Self-Perceived Health from 2008 to 2016 predicted more Sleep Disturbances (i.e., higher score) in 2016. On the other hand, less deterioration in Cognitive Functioning from 2008 to 2016 predicted poorer overall SQ (i.e., higher score) in 2016, and a smaller decrease in Social Participation from 2008 to 2016 predicted more Daytime Disturbances (i.e., higher score) in 2016.
Psychosocial variables including depressiveness and life satisfaction are known to be related to SQ (Brandolim Becker et al., 2018). Since they were not assessed in our study at all measurement points (in 2008 and in 2016), we could not enter them into the regression models. Therefore, we analyzed their Pearson correlations with the PSQI Total Score and seven domains. Depressiveness in 2008 was significantly positively correlated with PSQI Total Score and all PSQI domains except Daytime Disturbances. The lowest coefficients were observed for the Use of Sleep Medication (.16, p < .05) and Sleep Disturbances (.17, p < .05), and the highest for Total Score (.37, p < .001) and Subjective Sleep Quality (.46, p < .001). Life satisfaction in 2016 was significantly positively correlated with Sleep Disturbances (−.42, p < .001), Total Score (−.41, p < .001), Subjective Sleep Quality (−.39, p < .001), Sleep Latency (−.30, p < .001), and Use of Sleep Medication (−.26, p < .001). All of the observed relationships were in the expected direction, i.e., more depressiveness was associated with poorer SQ and greater life satisfaction with better SQ.
Discussion
This study examined SQ in older adults in Croatia, specifically those residing in nursing homes, and examined the associations of a set of different biological and psychosocial factors, assessed at two timepoints over the period of 8 years, with participants’ SQ assessed only at the last measurement point. We examined both the changes in cross-sectional and long-term contributions of the observed set of factors to the prediction of overall SQ and seven individual SQ domains.
Participants’ very old average age (77 years at baseline, and 84.5 years at the last measurement) is usually associated with a decline in health and in most functional aspects, as the expected age-related changes. The detected change in the observed psychosocial variables confirmed these decline, but the amount of change, with respect to the mean baseline results, varied from 3.6% (for Self-Perceived Health) to 13.8% (for Functional Ability). However, the participants were highly functional at the baseline measurement, which was a selection criterion for the participation in this study, and for the most part remained so 8 years later (Table 3). Even though the participants’ average functional ability and their average cognitive ability significantly decreased from baseline to final measurement point, their functioning could still be regarded as good. This is probably because these were the survivors of a much larger participants’ sample, the majority of whom had deceased in the meanwhile. Good cognitive and physical functioning enables people to work, socialize, and perform their social roles (Siedlecki, Salthouse, Oishi, & Jeswani, 2014).
Significant changes were found in the participants’ Self-Perceived Health over the 8-year period; however, it was on average rated as “good” at both measurement points, regardless of the high proportion of chronically ill participants (92%). This is the expected finding, confirmed by many studies. Self-perceived health is a well-researched psychological phenomenon, consistently not strongly correlated to older adults’ objective health. Older adults tend to perceive their health as good, unless chronic illness severely disrupts their functional ability (Idler, 1992; Smith, Young, & Lee, 2004).
The average frequency of social participation in participants was rated quite low at both measurements, and no significant change was found over the 8-year period. The associations of participation in social, leisure, and productive activities with positive emotional and physical outcomes have been confirmed (Berg et al., 2007, Kaliterna Lipovčan, Brkljačić, Prizmić Larsen, Brajša-Žganec, & Franc, 2018). Although most nursing homes offer a choice of social activities on the premises, the frequency of residents’ participation is usually low. More in-depth analyses of residents’ needs, and interest in specific social activities would be required in order to increase their social engagement.
Regarding participants’ depressive symptoms, even though this study did not consider the second measurement results from 2010 and depression was not assessed in 2016, it is important to note that the symptoms’ frequency increased from the first to the second measurement time, from a low to a moderate average score, indicating a mild decline in the participants’ mental health status. This finding is expected, even more so in older adults residing in institutions (Blazer, 2003).
Life satisfaction, introduced only at the final measurement time in 2016, was rated on average quite high. Other research found that institutions can provide a stable and supportive environment, which is important for older persons’ sense of subjective well-being (Asakawa, Feeny, Senthilselvan, Johnson, & Rolfson, 2009). Also, life satisfaction is regarded as an indicator of psychological adaptation in aging (Allerhand, Gale, & Deary, 2014; Baltes, 1997).
On average, overall SQ in participants was impaired, with 71% of participants scoring greater than 5 on the PSQI, which indicates poor SQ (Buysse et al., 1989). Since the same instrument was used, the finding is comparable to that reported by Valenza et al. (2013), who found a total PSQI greater than 5 in 72% of nursing-home residents – although this is higher than the usually reported frequency of poor SQ in older adults residing in institutions, which ranges between 50% (Azri et al., 2016) and 65% (Martin et al., 2010).
The results of other studies conducted so far did not provided definitive conclusions whether living in one’s own home was a protective factor for SQ of older adults. SQ in various forms of long-term care has been examined, but the types of facilities and level of support have usually not been described adequately. The terminology used may also differ between countries as well as in the level of care provided (e.g., assisted-living facilities, nursing homes, elderly homes, retirement homes, continuing care retirement communities, assisted-care villages, etc.), which makes generalizations of the SQ outcomes associated predominantly with factors of living arrangement difficult.
As mentioned, contemporary approaches to sleep in older adults emphasize the importance of factors contributing to SQ impairment beyond the aging process per se (Bloom et al., 2009; Mander, Winer, & Walker, 2017; Miner & Kryger, 2017). In that respect, we examined the factors that may be contributing to the expected age-related changes in overall functioning as well as to the SQ impairments in this population. Because of the high prevalence of SQ impairment in our participants, we were interested in cross-sectionally analyzing the time-synchronous structure of SQ predictors as well as the effects of changes in these predictors on SQ in 2016 over the 8-year period from 2008 to 2016.
Cross-sectionally, in 2016, the observed set of predictors significantly contributed to the explanation of 7–13% of the PSQI variance. Better Functional Ability and better Self-Perceived Health were predictive of overall better SQ (i.e., lower PSQI Total Score). Better Functional Ability also predicted shorter Sleep Latency, and better Self-Perceived Health predicted better Subjective Sleep Quality, less Sleep Disturbances and less Use of Sleep Medication (i.e., lower scores on each particular domain). Another significant predictor of more frequent Use of Sleep Medication (i.e., higher score on this domain) was participants’ older Age. Our results support other findings on the association between different aspects of physical and mental health and SQ in older adults (Neikrug & Ancoli-Israel, 2010). Similar findings on the relationship between PSQI scores and functional ability, mental health, and social engagement were reported by Valenza et al. (2013), and between PSQI scores and self-rated health by Štefan et al. (2018).
Our long-term examination of independent variables from the first study wave in 2008 (baseline), and of the changes in results from baseline to final measurement (in 2016), which could significantly predict the PSQI assessed in 2016, showed that the predictor variables from 2008 and the change in results from 2008 to 2016 significantly contributed to the explanation of 12–18% of variance of PSQI assessed in 2016. The amount of explained variance was bigger than in the cross-sectional models, which points to the importance of the change variables’ contribution in the prediction of the participants’ current SQ. The selected set of predictors explained a relatively small but significant amount of variance in Total Score, Subjective Sleep Quality, Sleep Disturbances and Daytime Disturbances. Among individual predictors, Self-Perceived Health in 2008 was again the strongest predictor of SQ in 2016. Better overall SQ, shorter Sleep Latency and less Sleep Disturbances (i.e., lower scores) in 2016 were found in those who estimated their health in 2008 as better. Unlike in the cross-sectional models, Functional Ability in 2008 was not a significant individual predictor in long-term analyses. However, bigger change in Functional Ability (i.e., bigger deterioration) from 2008 to 2016 significantly predicted overall worse SQ in 2016 (i.e., higher score), and the similar association was found for the change in Self-Perceived Health (i.e., bigger deterioration in Self-Perceived Health was associated with more Sleep Disturbances). On the other hand, smaller deterioration, but still deterioration, in Cognitive Functioning between two measurement points predicted poorer overall SQ in 2016, a finding that calls for further exploration, and smaller decrease, but still decrease, in Social Participation from 2008 to 2016 predicted more Daytime Disturbances in 2016 (i.e., higher score). In our study, global cognitive functioning was assessed and analyzed. In other studies, it has been confirmed that global cognitive functioning may not be associated with self-reported SQ impairment, both in high-functioning older adults, and in those with different chronic conditions (Cleutjens, Pedone, Janssen, Wouters, & Incalzi, 2016; Stavitsky, Neargarder, Bogdanova, McNamara, & CroninGolomb, 2011). More often, the effects of sleep problems on the variability in cognitive performance in older individuals seem to be restricted to specific cognitive domains. Also, some other factors’ effects (e.g., depression) may account for the variability in older individuals’ cognitive performance in relation to their SQ (Nebes, Buysse, Halligan, Houck, & Monk, 2009).
These findings show differences in the structure of SQ predictors between cross-sectional and long-term assessments, indicating how deterioration in different aspects of psychophysiological functioning may contribute differently to current SQ in residents of nursing homes.
In our study, Age was generally not a significant factor predicting SQ in a sample of very old institutionalised adults, which is consistent with other studies’ findings. A well-known meta-analysis of polysomnographic studies with healthy older adults by Ohayon et al. (2004) reported no age-related changes in SQ after the age of 60, except for the sleep efficiency parameter that continuously kept declining. In a study on a very big German sample of community-dwelling adults from 18 to 80 years, Hinz et al. (2017) reported a similar significant age trend in the increase of the mean PSQI Total Score from age groups ≤ 39 to 50–59, but not after that age. On the other hand, Zeitlhofer et al. (2000) reported that SQ assessed by the PSQI slowly but continuously deteriorated across age. The authors also demonstrated age-related changes in different aspects of the quality of life in the same direction. When examining the relationship between specific aspects of quality of life and SQ, Zeitlhofer et al. (2000) also showed that Physical Well-being and Emotional Well-being were significant factors contributing to SQ when all the relevant factors, including age, were controlled for.
The only exception with Age was, however, that it was a significant predictor of the Use of Sleep Medication in 2016. In our study, 54% of participants were taking some kind of sleep medication, which is similar to the results of Valenza et al. (2013). The use of sleep medication, especially benzodiazepines, in treating problems with sleep initiation and maintenance in older adults is frequent, and at the same time in contrast with the general recommendation of treatment options for sleep problems (Bloom et al., 2009; van de Straat, Buffel, & Bracke, 2018). Nonpharmacological treatment, including environment optimization, sleep hygiene education and interventions, and CBT-I is strongly recommended, as well as other types of hypnotics, since the use of benzodiazepines may be risky in older population due to the side effects related to sedation, dizziness, disorientation and muscle hypotonia (Bloom et al., 2009; van de Straat et al., 2018; Ye & Richards, 2018). Generally, diagnostics and treatment options should be carefully conducted, designed and implemented, and all health care providers included should be well informed in the field of sleep, since the necessary pharmacological treatment of primary morbidities can also be detrimental to SQ of older adults (Ancoli-Israel, 2011; Bloom et al., 2009, Ye & Richards, 2018).
The contribution of our study and its findings could be referred to the study participants. They were a very old group, but quite well-functioning – physically, cognitively, and psychologically, despite their chronic health conditions. Their characteristics, associated to their impaired SQ, clearly point to the risk factors that are recognizable and treatable. The results of our study also indicate that good mental health is related with SQ in older adults. Therefore, like in any other age group, interventions aiming at the protection of psychological wellbeing in older adults seem to be a valid and welcome option when we consider the improvement of their SQ as well. More so, education of professionals providing care at the long-term care facilities in the early detection of risk factors, and implementation of appropriate interventions are crucial. Namely, sleep hygiene education programs would additionally empower members of the management and staff to better understand sleep related issues in older adults, and to implement relatively simple interventions, like optimization of the physical environment by reducing light and noise at night-time, keeping the adequate room temperature, keeping aisles to bathroom clear from physical obstacles to reduce risk of night-time falls, pairing roommates according to their sleep related habits and needs, limiting residents’ daytime napping and reducing time in bed whenever possible to increase their sleep efficiency, increasing exposure to daylight in the morning hours or applying bright light therapy to reduce their night-time sleep latency, and introducing light exercise and physical activity. Also, discussing type and duration of pharmacological treatment with general practitioner, and applying non-pharmacological programs to improve residents’ SQ would be important to offer whenever possible. Educating the residents themselves about the factors affecting their SQ could have a positive impact, giving them a sense of more control over their lives, possibly also motivating them to take more advantage of various educational and psychosocial programs offered at the institution to increase their social engagement, improve their mental health, their quality of sleep and their quality of life in general.
Our future studies aim at comparing SQ between nursing-home residents and a comparable group of community-dwelling older adults. It would also be important to explore the knowledge and attitudes of various professionals involved in care for older adults regarding the specificities of their sleep-related needs and issues.
Limitations
Even though our results provide some insight in the long-term perspective on factors that might influence SQ in older adults, caution is required and no causal inferences can still be proposed.
The relationship between sleep problems and other health outcomes is complex, and the reverse causality cannot be ruled out (Gu et al., 2010). Inadequate sleep of poor quality is known to affect human functioning in many complex ways, and therefore it can also contribute to health deterioration in a population of older adults (Ye & Richards, 2018).
Also, the participants’ sample was not representative of the Croatian older population, so the generalisation of findings is limited. The SQ assessment was introduced only at the last measurement, so no control for prior levels of SQ was available. At the last measurement point, the depression symptoms assessment was substituted by the life satisfaction assessment which made impossible the inclusion of these important indices of psychological functioning in either model predicting the SQ.
Another caution refers to the reflection of participants’ high functioning in slightly negatively skewed result’s distributions in majority of variables. Since there were not extreme skewness cases, selected analyses were appropriately used.
The issue of low reliability of the last two PSQI domains was also present. It is possible that other associations would be found in regression models in which the PSQI Total Score would be calculated on the basis of only five domains.
All assessment instruments were self-report measures, and it is possible that other forms of assessment (sleep diaries, actigraphy, polysomnography) would have led to different conclusions.
Conclusions
SQ in 167 older adults in Croatia residing in nursing homes was assessed in 2008 and in 2016, and poor SQ was found in 71% of participants. By specific domains, Subjective SQ and Daytime Functioning were relatively good, while Sleep Efficiency was expectedly impaired the most. Despite a slight decline, the overall functioning of older persons in this research remained fairly good on average, over the 8-year period.
A set of biopsychosocial factors predicted between 7–13% of the SQ variance cross-sectionally, and between 12–18% of the SQ variance long-term, i.e., by status in psychophysical functioning 8 years earlier and the changes in the examined predictors over that period. Older Age did not significantly predict SQ, except for more frequent use of sleep medication in a cross-sectional model. Individual long-term predictors of SQ in 2016 were Self-Perceived Health in 2008, Cognitive Functioning in 2008, and changes in Cognitive Functioning, Self-Perceived Health, Functional Ability and Social Participation between 2008 and 2016. Although not entered in the regression models, Depressive Symptoms and Life Satisfaction showed consistent correlations with PSQI total Score and most of the individual PSQI domains.
Institutionalization is a risk factor for SQ impairments only partly due to the environmental factors, and to a greater extent due to the factors that may lead to the institutionalization, including the person’s mental health status. The findings of our research implicate the importance of longitudinal follow-up of changes in biological and psychosocial status of older adults, in order to timely address the relevant health-related issues that might adversely impact the quality of their sleep and, consequently, the overall quality of their lives.
We thank our colleagues Biserka Ross (ex Radošević-Vidaček), Ph.D., and Marija Bakotić, Ph.D., former employees of the Institute for Medical Research and Occupational Health, for their initiative and participation in the PSQI translation into Croatian. We also thank our colleague Nina Lučanin for her participation in the scale translation.
References
(2014). The dynamic relationship between cognitive function and positive well-being in older people: A prospective study using the English Longitudinal Study of Aging. Psychology and Aging, 29, 306–318. https://doi.org/10.1037/a0036551
(2011).
Sleep medicine in the elderly . In H. MeirM. H. KrygerT. RothW. C. DementEds., Principles and practice of sleep medicine (5th ed., pp. 1524–1543). St. Louis, MI: Elsevier Saunders.(2009). Do the determinants of health differ between people living in the community and in institutions? Social Science and Medicine, 69, 345–353. https://doi.org/10.1016/j.socscimed.2009.05.007
(2016). Sleep quality among older persons in institutions. Procedia - Social and Behavioral Sciences, 234(31), 74–82. https://doi.org/10.1016/j.sbspro.2016.10.221
(1997). On the incomplete architecture of human ontogeny: Selection, optimization, and compensation as foundation of developmental theory. American Psychologist, 52, 366–380.
(2012). Validation of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older black and white women. Sleep Medicine, 13(1), 36–42. https://doi.org/10.1016/j.sleep.2011.04.005
(2007).
A developmental approach to psychosocial risk factors and successful aging . In C. M. AldwinC. L. ParkA. SpiroEds., Handbook of health psychology and aging (pp. 30–53). New York: Guilford.(2003). Depression in late life: Review and commentary. Journal of Gerontology: Medical Sciences, 58, 249–265. https://doi.org/10.1016/0165-1781(89)90047-4
(2009). Evidence-based recommendations for the assessment and management of sleep disorders in older persons. Journal of the American Geriatrics Society, 57, 761–789. https://doi.org/10.1111/j.1532-5415.2009.02220.x
(1990). Linear statistical models: An applied approach (2nd ed.). Belmont, CA: Duxbury.
(1995). Measuring disease. Buckingham, UK: Open University Press.
(2018). Depression and quality of life in older adults: Mediation effect of sleep quality. International Journal of Clinical and Health Psychology, 18(1), 8–17. https://doi.org/10.1016/j.ijchp.2017.10.002
(1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213.
(2010). Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep, 33, 585–592. https://doi.org/10.1093/sleep/33.5.585
(1998). Psychometric evaluation of the Pittsburgh Sleep Quality Index. Journal of Psychosomatic Research, 45(1), 5–13.
(2016). Sleep quality disturbances and cognitive functioning in elderly patients with COPD. European Respiratory Journal Open Research, 2(3), 00054–2016. https://doi.org/10.1183/23120541.00054-2016
(1984). Stari ljudi
[The old people] . Zagreb: Stvarnost.(2003). Iskustvo starenja
[The experience of ageing] . Jastrebarsko: Naklada Slap.(1997). The role of psychological factors in the aging process: Stress and health as predictors of aging. Croatian Medical Journal, 38, 222–227.
(2011). Cross-country variation in sleep disturbance among working and older age groups: an analysis based on the European Social Survey. International Psychogeriatrics, 29, 1413–1420. https://doi.org/10.1017/S1041610211000664
(2004). Sleep disturbances and chronic disease in older adults: Results of the 2003 National Sleep Foundation Sleep in America Survey. Journal of Psychosomatic Research, 56, 497–502. https://doi.org/10.1016/j.jpsychores.2004.02.010
(2014). Insomnia in long-term care facilities: A comparison of seven European countries and Israel. The Services and Health for Elderly in Long Term Care Study. Journal of the American Geriatric Society, 62, 2033–2039. https://doi.org/10.1111/jgs.13099
(2010). Sociodemographic and health correlates of sleep quality and duration among very old Chinese. Sleep, 33, 601–610. https://doi.org/10.1093/sleep/33.5.601
(2017). Sleep quality in the general population: Psychometric properties of the Pittsburgh Sleep Quality Index, derived from a German community sample of 9, 284 people. Sleep Medicine, 30, 57–63. https://doi.org/10.1016/j.sleep.2016.03.008
(1992). Self-assessed health and mortality: A review of studies. International Review of Health Psychology, 1, 33–54.
(2018). Leisure activities and the subjective well-being of older adults in Croatia. GeroPsych, 31, 31–39. https://doi.org/10.1024/1662-9647/a000179
(2016). Biopsychosocial predictors of sleep quality in retirement home residents. 23rd Congress of the European Sleep Society, Bologna, Italy. Journal of Sleep Research, 25(1), 101.
(submitted). Sleep quality and daytime functioning in older European adults. Manuscript in preparation.
(2018). Sleep in normal aging. Sleep Medicine Clinics, 13(1), 1–11. https://doi.org/10.1016/j.jsmc.2017.09.001
(2017). Longitudinal psychosocial predictors of life satisfaction in old persons. 20th Psychology Days in Zadar, Book of Selected Proceedings (pp. 115–125). Zadar, Croatia: Department of Psychology, University of Zadar.
(2017). Sleep and human aging. Neuron, 94(1), 19–36. https://doi.org/10.1016/j.neuron.2017.02.004
(2010). Sleep quality in residents of assisted-living facilities: Effect on quality of life, functional status, and depression. Journal of American Geriatric Society, 58, 829–836. https://doi.org/10.1111/j.1532-5415.2010.02815.x
(2011). Poor self-reported sleep quality predicts mortality within one year of inpatient post-acute rehabilitation among older adults. sleep, 34, 1715–1721. https://doi.org/10.5665/sleep.1444
(2017). Sleep in the aging population. Sleep Medicine Clinics, 12(1), 31–38. https://doi.org/10.1016/j.jsmc.2016.10.008
(2016). The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and nonclinical samples: A systematic review and meta-analysis. Sleep Medicine Reviews, 25, 52–73. https://doi.org/10.1016/j.smrv.2015.01.009
(2009). Self-reported sleep quality predicts poor cognitive performance in healthy older adults. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 64, 180–187. https://doi.org/10.1093/geronb/gbn037
(2010). Sleep disturbances in nursing homes. The Journal of Nutrition, Health and Aging, 14, 207–211. https://doi.org/10.1007/s12603-010-0051-8
(2004). Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep, 27, 1255–1273. https://doi.org/10.1093/sleep/27.7.1255
(2001). How age and daytime activities are related to insomnia in the general population: consequences for older people. Journal of American Geriatric Society, 49, 360–366. https://doi.org/10.1046/j.1532-5415.2001.49077.x
(1996). CAPE - Clifton postupci za procjenu starijih osoba
[Clifton assessment procedures for the elderly] . Jastrebarsko: Naklada Slap.(2014). The relationship between social support and subjective well-being across age. Social Indicators Research, 117, 561–576. https://doi.org/10.1007/s11205-013-0361-4
(2004). Optimism, health-related hardiness and well-being among older Australian women. Journal of Health Psychology, 9, 741–752. https://doi.org/10.1177/1359105304045373
(2012). Reliability and validity of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older men. The Journals of Gerontology. Series A, 67, 433–439. https://doi.org/10.1093/gerona/glr172
(2009). Sleep quality and falls in older people living in self- and assisted care villages. Gerontology, 55, 162–168. https://doi.org/10.1159/000146786
(2011). The impact of sleep quality on cognitive functioning in Parkinson’s disease. Journal of the International Neuropsychological Society: JINS, 18(1), 108–117. https://doi.org/10.1017/S1355617711001482
(2018). Sleep duration and sleep quality as predictors of health in elderly individuals. Sustainability Journal, 10(11), 3918. https://doi.org/10.3390/su10113918
(2007). Using multivariate statistics. Boston: Pearson/Allyn & Bacon.
(2017). Guidelines for developing, translating, and validating a questionnaire in perioperative and pain medicine. Saudi Journal of Anaesthesia, 11(Suppl 1), S80–S89. https://doi.org/10.4103/sja.SJA_203_17
(2015). How well does Europe sleep? A cross-national study of sleep problems in European older adults. International Journal of Public Health, 60, 643–650. https://doi.org/10.1007/s00038-015-0682-y
(2018). Medicalization of sleep problems in an aging population: A longitudinal cross-national study of medication use for sleep problems in older European adults. Journal of Aging and Health, 30, 816–838. https://doi.org/10.1177/0898264317696775
(2013). Nursing homes: Impact of sleep disturbances on functionality. Archives of Gerontology and Geriatrics, 56, 432–436. https://doi.org/10.1016/j.archger.2012.11.011
(2000). Sleep and quality of life in the Austrian population. Acta Neurologica Scandinavica, 102, 249–257. https://doi.org/10.1034/j.1600-0404.2000.102004249.x
(2018). Sleep and long-term care. Sleep Medicine Clinics, 13(1), 117–125. https://doi.org/10.1016/j.jsmc.2017.09.011


