The Influence of Neuroticism on the Muscle Response in the Trapezius and Frontalis Muscles to Anticipatory Stress
Abstract
Abstract. Objective quantification of mental stress in the workplace would be beneficial for designing work tasks to avoid the negative consequences of mental stress. Methods such as surface electromyography have proven to be sensitive to mental demands. However, there is little knowledge about the muscle response and moderating factors during anticipatory stress paradigms. This study examined whether the personality dimension neuroticism moderates the muscle response to the expectation of an unpredictable electrical shock. Forty-seven subjects underwent three expectation phases, in which they could expect a pleasant audio signal (NoShock) or an electric shock in two conditions (anticipation of the first: Shock1, and second electric shock: Shock2) at an unpredictable moment. The frontalis muscle activity and the upper and upper/middle parts of the trapezius muscle were recorded using surface electromyography. Neuroticism was surveyed using the Big Five Inventory to assign the subjects to a group with lower or higher neuroticism. Shock1 only induced higher trapezius muscle activity in the higher neuroticism group, which vanished during Shock2, while the frontalis muscle showed no significant effects. The results suggest that neuroticism should be considered a moderating factor in assessing anticipatory stress using surface electromyography at the trapezius muscle.
Mental stress as a response to a stressor can have many short- and long-term implications for human beings. On the one hand, mental stress triggers acute physiological responses and may affectively influence behavior, mood, and well-being. On the other, chronic mental stress is associated with cardiovascular and musculoskeletal complaints or mental disorders (Esler, 2017; Lundberg, 2002; Schneiderman et al., 2005). In particular, work-related mental stress counts as a risk factor for various health problems, including musculoskeletal disorders, leading to incapacity for work and early retirement (Harvey et al., 2017). Several environmental and organizational exposure factors at the workplace, such as time pressure or high workloads, are identified as workplace stressors (Chamoux et al., 2018), which usually occur repeatedly over a long period of time and are perceived with low to moderate intensity of mental stress (Darvishi et al., 2016; Lowndes et al., 2018). From this perspective, it would be beneficial to objectively quantify mental stress by acquiring the acute physiological response to workplace stressors before, during, or after particular work tasks to develop concepts at the workplace to avoid negative consequences of mental stress.
As measured by surface electromyography (sEMG), the muscle response appears sensitive to various mental stress paradigms. Driven by the assumption that musculoskeletal complaints at the neck and shoulder could be caused by mental stress, many studies have investigated the activity of the trapezius muscle at monitor-based workplaces. These studies showed that several muscles are sensitive to mental stress caused by cognitive or psychosocial demands in the sense of increased muscle activity (Eijckelhof et al., 2013; Lundberg et al., 2002; Shahidi et al., 2013). Therefore, the application of sEMG might prove useful in identifying mental stress at the workplace. However, sEMG is rarely used in conjunction with anticipatory stress, potentially triggering feed-forward controlled stress responses (Del Giudice et al., 2018; Ursin & Eriksen, 2010). At the workplace, anticipatory stress can be related to an important presentation or the evaluation of the working performance by a supervisor in the future. For this particular type of mental stress, it is unclear whether the muscle response changes after repeated exposure and how long recovery reactions would take. Further, since the upper trapezius muscle is rather sensitive to external disturbances, for example, movement artifacts, it may be helpful to identify other muscle areas that are both sensitive to different manifestations of mental stress and less involved in movements of the upper body. Studies of facial expressions, for example, linked specific muscles with major emotions like anger, disgust, fear, happiness, sadness, and surprise (Chen et al., 2016; Ekman & Oster, 1979). Borges et al. (2018) and Perkins et al. (2012) found that potential threat scenarios produce environmental scanning facial behavior that in turn could result in increased activation of the frontalis muscle to broaden the field of vision.
Furthermore, the muscle response to workplace stressors could be moderated by individual predispositions as assessed by personality dimensions. In their Five-Factor model of personality, Costa and McCrae (1987) described the personality dimension neuroticism as a tendency to experience negative, distressing emotions and to possess associated behavioral and cognitive traits. Research has shown that a higher level of neuroticism is related to a higher appraisal of negative affect (Penley & Tomaka, 2002) or a modified sympathetic and hypothalamic-pituitary-adrenal reactivity (Lahey, 2009). In an experimental study, McCleery and Goodwin (2001) showed that among 28 young adults, lower neuroticism led to a stronger cortisol response after a combined dexamethasone-corticotropin-releasing hormone test than higher neuroticism. Another study observed a greater skin conductance response after an emotional attending task among subjects with higher neuroticism than lower neuroticism (Reynaud et al., 2012). In addition, the degree of neuroticism may affect the recovery time after mental stress and the physiological response during repeated exposures to a stressor (Hughes et al., 2011).
The primary objective of the present study was to determine the muscle response of the trapezius and frontalis muscles and the moderating role of neuroticism during the expectation of an unpredictable and potentially painful electric shock compared to the expectation of a pleasant audio signal and how this response changes in repeated exposure. We hypothesized that muscle activity increases during the expectation of the first electrical shock compared to the audio signal and that the increase is moderated by neuroticism. In case of increased muscle activity, we further expected an adaption effect with a decrease in muscle activity during the expectation of the second electric shock compared to the first electric shock. The secondary objective was to assess muscle activity during a four-minute recovery phase after the first expectation of the electric shock in the event of significant muscle response. We expected a decrease during the recovery phase that could be impaired in the group with higher neuroticism.
Materials and Methods
Subjects
The estimated sample size of 60 subjects was used in this explorative study to investigate the research objectives. This sample size estimation was inspired by a previous study (Luijcks et al., 2014), which included 64 subjects and found increased muscle activity at the upper part of the trapezius muscle in response to the expectation of an electrical shock by using a similar mental stress paradigm in combination with a similar research question. We intended to have a balanced distribution of gender (males and females) and three age groups (18–34, 35–51, 52–67 years). Due to restrictions in time and resources, we recruited 53 eligible healthy subjects. Exclusion criteria were self-reported acute pain or complaints at the neck or shoulder area, neurological or psychological diseases, under 18 or over 67 years of age, and using medication including beta-blockers, analgesics, antipsychotics, antidepressants, anticonvulsants, or anxiolytics. All subjects received financial compensation. The study was approved by the local ethics committee of the Medical Faculty of the University of Tübingen (561/2016BO1) and was conducted according to the principles of the Declaration of Helsinki (version 2013, Fortaleza).
Procedure
E-mail distributors recruited subjects among personnel of the University Hospital and University of Tübingen (Germany), and personal contacts or via friends and relatives. Potential subjects were informed to avoid sports activities involving the upper body and alcohol consumption one day before the measurement, caffeine and cocoa consumption the day of measurement, and eating or drinking (except water) at least two hours before the measurement. All measurements took place during one appointment that lasted about two hours.
Before the experiment, eligible subjects gave their written informed consent and filled out the Big Five Inventory-SEOP (Gosling et al., 2003; see Neuroticism section). Afterward, they were prepared for the sEMG measures (see Surface Electromyography section). The measurements were carried out in a 4 × 4 m noise-isolated room, in which light and temperature (22–25 °C) conditions were held constant. During the measurement, subjects were seated on a chair in front of a desk with a screen. Posture and position were individually adjusted to control for body height. The top edge of the screen was aligned to the eyes of the subjects. Subjects were instructed to find a comfortable sitting position. During the recordings, the subjects had to look at the screen, sit still, and place their hands on the desk with their palms turned upwards while their shoulders were relaxed. A room divider prevented visual contact between the subject and examiner, and experimental guidance was provided through a standardized slide show presented on a screen in front of the subjects (see Instructions to the Subjects section). After every slide, subjects had to confirm verbally that the information about the procedure was understood.
The experiment included three conditions in a predefined order: the first control condition without an electrical shock (NoShock), the second (Shock1), and the third condition (Shock2) with an electrical shock. During the NoShock condition, a pleasant audio signal, which was introduced to the subjects in advance, was used instead of the electrical shock. Shock2 was a repetition of Shock1 with a one-minute break in between. Directly before each condition, subjects had to rate their mental stress (see Perceived Mental Stress section). For all three conditions, subjects were not informed about the exact time point of the stimulus (audio signal or electrical shock). Directly after NoShock and before the Shock1 and Shock2 conditions, the individual electrical stimulus to induce physical pain (pain threshold) was determined (see Electric Shocks section).
Each condition consisted of an expectation phase, a stimulus, and a recovery phase, as visualized in Figure 1. The duration of the expectation phase was 1 min 55 s for NoShock, 2 min 55 s for Shock1, and 2 min 25 s for Shock2. Different durations for the expectation phase were chosen to prevent subjects from anticipating the onset of the electrical shock. The expectation phase was subdivided into 3, 4, or 5 periods of 30 s plus a final period of 25 s directly before the stimulus. During each period, the subjects observed a rectangle as displayed on the monitor in front of them filled with incremental steps of 5 s and were full after 25 s, after which the filled rectangle remained visible for another 5 s. The final period always ended after 25 s with the application of the audio signal or the electrical shock at the time when the completely filled rectangle appeared.
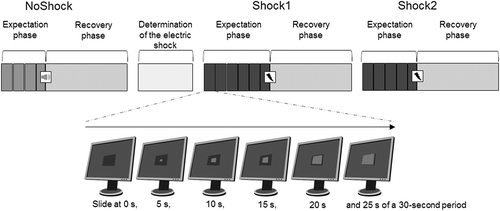
Instructions to the Subjects
Before the expectation phase, subjects were briefed that a computer program would trigger the stimulus once within the next 4 min at an unforeseeable moment, but only during the time span within which the rectangle was completely filled. For Shock1 and Shock2, subjects were informed that the intensity of the electrical shock would be randomly determined by the computer program and would lie somewhere between their sensation threshold and clearly above their pain threshold (see Electrical Shocks section). The subsequent recovery phase lasted 4 min.
Electrical Shocks
The intensity of the electrical shock applied during Shock1 and Shock2 was individually determined based on equation 1.
Equation 1 was adopted from previous literature studies (Luijcks et al., 2014; Vossen et al., 2011) and slightly modified to ensure that the electrical shock was experienced as painful but still acceptable.
A two-step process determined the sensation and pain thresholds. In the first step, electrical shocks were applied to start from 0 mA in incremental steps of 0.4 mA until the subject felt the shock. This point was defined as the sensation threshold. In the second step, electrical shocks were applied to start from the sensation threshold in incremental steps of 1.0 mA until the subject reported it was painful. This point was defined as the pain threshold. This procedure was performed twice, and the mean of both thresholds (mA) was used in equation 1.
The electrical shocks were rectangle pulses of 2 ms duration applied by a constant current electro-stimulator (DS7A, Digitimer Ltd., UK) through Ag/AgCl cup electrodes filled with conductive electrode paste (Ten20, Weaver and Company, USA) that were placed at the distal phalanx of the index and middle finger of the non-dominant hand using adhesive tape (Fixomull stretch, BSN medical, Germany).
Surface Electromyography
The skin was prepared with abrasive paste (Nuprep, Weaver and Company, USA) to improve signaling for the bipolar sEMG measurement. Disposable surface electrodes (H93SG, Covidien, Kendall, USA) with an active area of 15 mm diameter were placed with an inter-electrode distance of 25 mm parallel to the muscle fibers at three different muscles or muscle sections at the dominant half of the body. Positions were: (1) the upper part of the trapezius muscle with the midpoint between the 7th cervical vertebrae and the acromion (TRAPUP), (2) the transition between the upper and middle part of the trapezius muscle with the midpoint between the 7th cervical vertebrae and the superior angle of the scapula (TRAPUP/MID), and (3) the frontalis muscle with the outer edge of the lower electrode above the eyebrow on the vertical line through the midpoint of the eyes (FRONT). The ground electrode was placed at the 7th cervical vertebrae.
The bipolar sEMG signals were differentially amplified, filtered (high pass filter, 2nd order, −3 dB at 4 Hz; low pass filter, 11th order, −3 dB at 1,300 Hz), sampled at 4,096 Hz, analyzed, and stored using a combined data analyzer and logger (PS11-UD, THUMEDI GmbH & Co. KG, Germany; overall CMRR > 96 dB; the overall effective sum of noise < 0.8 μV RMS; linearity typ. ± 0.15 dB at 25–1,100 Hz). Data were real-time transformed by the device into the frequency domain (1,024-point Fast Fourier Transformation using a Bartlett-window with 50% overlap) and digitally filtered (high-pass filter, 11th order, –3 dB at 80 Hz to avoid interference from heart frequencies and motion artifacts). Powerline interference (50 Hz and its first seven harmonics) was removed by replacing it with the spectral values of a 4-Hz wide band around its center frequency using both spectral neighbors. The root-mean-square of the electrical activity (EA [μV]) was real-time calculated (375 ms moving window with 50% overlap) from the power spectrum and stored synchronously to the raw data by the PS11-UD device. The recorded EA was processed with custom-made software (SABCOM, Institute of Occupational and Social Medicine and Health Services Research, University Hospital Tübingen, Germany). The EA time series of each subject was screened for inconsistencies. If inconsistencies were suspected, photos synchronously taken every 2 s throughout the measurement were used to verify whether this could be related to violations of the procedure requirements (i.e., closed eyes, speaking, or apparent body movements). Sequences of the EA signal affected by violations of the measurement procedure were excluded, and 0.5 s before and after applying the electrical shock or the audio signal.
After visual inspection of the EA signals, the EA was normalized to a reference voluntary electrical activity (EARVE [1]). Reference voluntary electrical activities were determined during submaximal isometric muscle contractions lasting about 15 s, of which the most stable 10 s were used to calculate the median EA. Subjects were asked to abduct their arms to 60° in the scapular plane for the trapezius muscle and open their eyes as wide as possible by lifting the eyebrows for the frontalis muscle.
The average of the normalized EA of selected periods (Table 1) was calculated for each condition. The two periods of the expectation phase were chosen to account for a possible increase or decrease in muscle response over time. The study by Luijcks et al. (2014) investigated a recovery phase of only 2 min. Therefore, we decided to apply a recovery phase of 4 min divided into four periods to find out whether the suggested decrease in muscle activity continued after 2 min.

Perceived Mental Stress
Subjects rated their perceived mental stress on an 11-point numeric rating scale immediately before the start of each condition by answering the following question that was displayed on the monitor: “How would you rate your current mental tension on a scale from 0 to 10, when 0 means no mental tension and 10 the highest imaginable mental tension?”. Perceived mental stress was assessed as a subjective measure of a stress response to verify the current paradigm of mental stress.
Neuroticism
Subjects rated their personality dimension neuroticism by answering the Big Five Inventory-SEOP (Gosling et al., 2003; Schupp & Gerlitz, 2014). Based on the construction of three questions, the mean score ranged from 1 to 7 for every subject. Internal consistency of neuroticism was acceptable with a Cronbach’s α level of .779 in the present sample. If subjects rated neuroticism lower than or equal to the median of the overall distribution found in our study sample, they were allocated to the lower-neuroticism group. For values higher than the median, subjects were allocated to the higher-neuroticism group.
Statistical Analyses
Presence of Mental Stress
To confirm the presence of mental stress, two-factor mixed analysis of variance (ANOVA) was applied to the mental stress ratings, with condition (3 levels) as the within-subject factor and neuroticism (2 levels) as the between-subject factor. Additionally, EARVE during the expectation phase (mean of EP1 and EP2) was correlated with the ratings of perceived mental stress before the Shock1 condition using the Spearman rank correlation rs to clarify whether a significant muscle response matched the perception of mental stress.
Primary Objectives
To assess whether a possible muscle response to the anticipated stressor and its repetition depends on the personality dimension neuroticism, two-factor mixed ANOVA with the within-subject factor condition (3 levels) and the between-subject factor neuroticism (2 levels) was applied to the dependent variables of muscle activity (TRAPUP, TRAPUP/MID, and FRONT) during the expectation phase (mean of EP1 and EP2). Since the muscle activity was not normally distributed, it was logarithmized (lnEARVE). Further, the assumption of homogeneity of variance was violated, and an explorative approach was chosen. Separately for each neuroticism group, single-factor repeated measures ANOVAs were applied to the dependent variables of the muscle activity with the within-subject factor condition (3 levels).
Secondary Objective
To assess the recovery effects after a muscle response, single-factor repeated measures ANOVAs were performed for the dependent variables of the muscle activity within each neuroticism group if post hoc analyses revealed a significant difference between the conditions NoShock and Shock1. The periods of the recovery phase (4 levels: RP1, RP2, RP3, RP4) served as the within-subject factor time. Since the muscle activity was not normally distributed, it was logarithmized.
Before the ANOVAs, all dependent variables were checked visually for normal distribution, sphericity using Mauchly’s sphericity test and, if necessary, for the assumption of homogeneity of variances using Levene’s test. The standardized partial eta squared (ηp2) was calculated for the main and interaction effects of the ANOVAs. Post hoc analyses were performed using Tukey’s honestly significant difference tests. The level of significance was set at α = .05. The statistical analyses were carried out using SPSS 26 (IBM, USA) and JMP 13 (SAS Inc., USA).
Results
Of the 53 recruited subjects, two dropped out due to discomfort, and four were excluded retrospectively: two due to protocol violations and two due to signal interference. Thus, the final dataset included 47 subjects (28 women and 19 men; age 34 ± 14 years, height 174 ± 10 cm, weight 70 ± 14 kg).
Presence of Mental Stress
The result of the two-factor mixed ANOVA applied to the mental stress ratings showed a significant main effect of condition [F(1.6, 74.0) = 28.5, p < .001, ηp2 = .39], but not of neuroticism [F(1.0, 45.0) = 0.5, p = .505, ηp2 = .01], and there was no significant interaction between condition and neuroticism [F(1.6, 74.0) = 0.3, p = .690, ηp2 = .01]. Post hoc comparisons of the main effect of condition revealed a significantly higher mental stress rating directly before the expectation phase of Shock1 versus NoShock (p < .001) and Shock2 (p = .006). In addition, the mental stress rating before the Shock2 condition was significantly higher than before NoShock (p < .001; Figure 2).
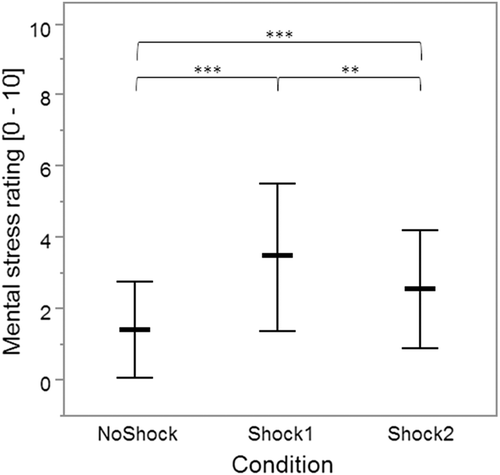
The mental stress rating before the expectation phase of Shock1 and the muscle activities during the subsequent expectation phase did not correlate (TRAPUP: rs = −0.067, p = .653; TRAPUP/MID: rs = 0.008, p = .960; FRONT: rs = −0.076, p = .611).
Primary Objectives
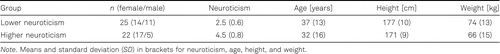
After the total sample was separated using a median split of neuroticism at a score of 3.3, lower and higher neuroticism groups were characterized by the anthropometric data in Table 2.
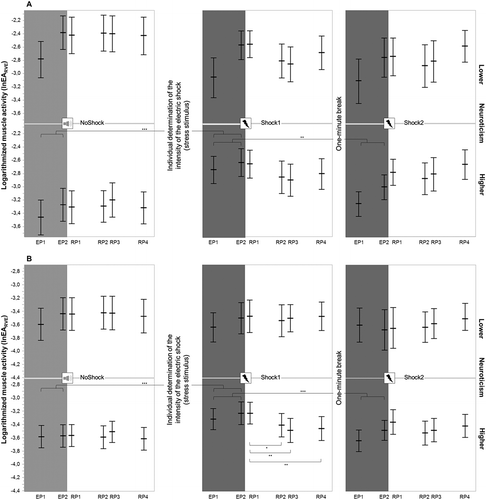
The single-factor repeated measures ANOVAs indicated significant main effects of condition, but only within the higher neuroticism group for the muscle activity of TRAPUP and TRAPUP/MID (Table 3). The post hoc comparisons showed higher muscle activity in both muscles during Shock1 versus NoShock (TRAPUPp < .001; TRAPUP/MID p < .001) or Shock2 (TRAPUPp = .004; TRAPUP/MIDp < .001) conditions, but not between NoShock and Shock2 (TRAPUPp = .503; TRAPUP/MIDp = .995) conditions (Figure 3).

Secondary Objective
As a significant muscle response was found in the primary analysis for the group with higher neuroticism, two single-factor repeated measure ANOVAs were applied to the muscle activity of TRAPUP and TRAPUP/MID during the recovery phase of the Shock1 condition for this group. Subsequently, a significant main effect of time was found for the muscle activity of TRAPUP/MID [F(1.3, 27.5) = 6.5, p = .011, ηp2 = .24], but not for TRAPUP [F(1.6, 33.1) = 2.8, p = .090, ηp2 = .12]. The post hoc comparisons for TRAPUP/MID showed significantly higher muscle activity during RP1 compared to RP2 (p = .033), RP3 (p = .001), and RP4 (p = .004, Figure 3).
Discussion
In the present study, an anticipatory stress paradigm that contained elements of unpredictability about a potentially painful threat in the form of an electrical shock was chosen to provoke a muscle response of the trapezius and the frontalis muscles. The neuroticism dimension of a short version of the Big Five Inventory included neuroticism as a potentially important personal modifying factor in the model. The primary objectives of this study were to investigate the moderating role of the personality dimension neuroticism on potentially elevated muscle activity at two parts of the trapezius muscle and the frontalis muscle during the expectation of an initial electrical shock, as well as possible adaptation effects of the muscle response when the mental stress paradigm was experienced for the second time. The second objective was to investigate the recovery phase after the first application of the electrical shock and the influence of neuroticism. The research paradigm of provoking anticipatory stress seemed to be fulfilled since low to moderate perceived mental stress was reported in the subjective evaluations.
Since the statistical prerequisite of homogeneity of variances was not fulfilled for the planned mixed ANOVAs, single-factor repeated measures ANOVAs were used separately for the two neuroticism groups. Consequently, a potentially moderating influence of neuroticism on the muscle response to the present stress paradigm could not be statistically proven. However, in contrast to the frontalis muscle, where significant effects of the expectation of an electrical shock were absent, the muscle activity of the trapezius muscle showed a significant response during the first expectation phase, but only in the group with higher neuroticism. Additionally, during the four-minute recovery phase, the muscle activity of the upper/middle part of the trapezius muscle decreased not further after 180 s. These findings align with the current literature, which suggests that neuroticism can modify physiological reactivity during mental stress. However, results from the literature are not entirely conclusive about a positive linear relation between the physiological response during mental stress and the level of neuroticism (Lahey, 2009).
One aspect that may have contributed to the trapezius muscle response of the group with higher neuroticism is that in the present study, the mental stress paradigm of anticipatory stress focused on the expectations of a stressor (electrical shock) as well as on the expectation of possible aversive effects of the stressor (pain). Since neuroticism is associated with increased sensitivity to threats and susceptibility to negative thoughts and emotions (Perkins et al., 2015), a more negative appraisal of the stressor could have lowered the threshold for response triggered. Further, elements of unpredictability in a threat scenario can trigger emotional responses with feelings of anxiety (Grillon et al., 2004; Grupe & Nitschke, 2013; Tracy et al., 2017). Since individuals with higher neuroticism are more likely to experience feelings of anxiety (Kendler et al., 2004), it is possible that the emotional processing of anxiety may have influenced a behavioral response, and that may explain the differences between groups (Li et al., 2019).
Interestingly, despite elevated muscle activity within the group with higher neuroticism during Shock1, there were no correlations to the perceived mental stress. A discrepancy between perceived mental stress and the corresponding physiological response was also found by Campbell and Ehlert (2012) and emphasized the advantage of using additional objective methods in the context of a mental stress assessment.
Furthermore, both parts of the trapezius muscle showed an adaptation effect during the expectation of the second electrical shock within the group with higher neuroticism, indicated by reduced muscle activity. In contrast, Willmann et al. (2012) found no adaption effects of the upper trapezius muscle during a Stroop color word test in healthy subjects. However, they related their findings to a high attentional load during the test, which plays a subordinate role in the present mental stress paradigm.
The lower neuroticism group showed no significantly increased muscle activity of the trapezius muscle during the expectation of an electrical shock. Consequently, no adaptation effects were observed at the second exposure to the same anticipatory stress or subsequent recovery phases. Given a mean value of 2.6 for the personality dimension neuroticism in a sample representative of the German population, the median value of 2.5 cannot be interpreted as low (Bluml et al., 2013), and shows that there is most likely no bias in the present study sample towards a low level of neuroticism.
However, previous research reported elevated muscle activity at the upper part of the trapezius muscle in response to a comparable anticipatory stress paradigm (Luijcks et al., 2014, 2016), without distinguishing between higher and lower neuroticism. These studies used a continuous three-minute period, during which the electrical shock was potentially applied, while the effective expectation-time in the present study accumulated up to 25 s (Shock1) and 20 s (Shock2), respectively, and may account for weakened mental stress. Nonetheless, the mental stress ratings collected directly before the shock conditions were comparable to those mentioned by Luijcks et al. (2014) (on mean 3.6). Another difference concerns the instructions given to the subjects during the expectation phases. In the current study, subjects tracked a rectangle on a screen, whereas Luijcks et al. (2014) reported that subjects only had to keep their eyes open. Therefore, it is possible that effects on muscle activity were masked by the effects of the visual demands (Richter et al., 2010; Zetterberg et al., 2013).
Moreover, there are several studies showing associations between various mental stress paradigms and elevated muscle activity at different parts of the body (Krantz et al., 2004; Lundberg et al., 1994, 2002; Nilsen et al., 2007; Westgaard et al., 2013; Woda et al., 2013). However, a notable difference to the present study with respect to the anticipatory stress paradigm should be mentioned. In the present study, subjects were positioned in a resting posture, while subjects in the aforementioned studies were involved in tasks requiring cognitive and motor activities, such as the Trier Social Stress Test, arithmetic calculations, or the Stroop color word test. In these studies, cognitive tasks were combined with executive functions, which were accompanied by elements of social assessment. The social assessment can lead to additional psychosocial stress, which may account for a stronger physiological response (Dickerson & Kemeny, 2004). Supporting this hypothesis, D’Attilio et al. (2013), for example, presented emotionally affective pictures while subjects were standing comfortably and were not involved in cognitive or motor tasks. Their results indicated no differences in sEMG amplitudes between neutral, pleasant, or unpleasant pictures at the upper part of the trapezius muscle and some facial muscles.
A limitation of this study may be attributed to “invisible” postural changes during the measurement procedure. Postural changes were assessed visually by screening pictures of the subjects for violations of the procedure requirements. However, it cannot fully be ruled out that slight head movements or variations of the angle between upper and forearm caused variability in the activity at the trapezius muscle across and within conditions (Goncalves et al., 2017), especially when considering low levels of muscle activity at the trapezius muscle (total mean and standard deviation of the non-normalized EA of TRAPUP 2.91 ± 3.98 and TRAPUP/MID 2.36 ± 2.26 μV).
The difference between the gender ratios in lower and higher neuroticism groups seems to indicate that the personality dimension neuroticism is more emphasized in women, which is supported by literature (Costa et al., 2001). Therefore, the different responses of the muscle activity of the trapezius muscle of the two groups could reflect a different physiological response during mental stress of men and women, due to sex-specific brain anatomy or hormonal status (Novais et al., 2017). Juster et al. (2016), for example, found similar cortisol responses to the Trier Social Stress Test in women and men after adjusting for sex hormone, and Childs et al. (2010) showed that women in the luteal phase of the menstrual cycle had a stronger heart rate response to the Trier Social Stress Test than women in the follicular phase.
It should be noted that the generalizability of the selected paradigm regarding anticipatory stress in the workplace is limited. On the one hand, this results from the threat scenario of anticipated pain due to the expectation of an electric shock, which can hardly be found in the working environment. On the other hand, the subjects were asked to sit still without any possibility to avoid or mitigate the anticipated consequences of the stressor by their own actions. Accordingly, both existing paradigms, such as the Trier Social Stress Test (Kirschbaum et al., 1993) and newly developed paradigms that allow for a more realistic approach to real scenarios in the working environment would have to be considered for better generalizability in further studies.
Conclusion
The analysis of the present study showed in two groups, with lower and higher neuroticism, different responses of the trapezius muscle during anticipatory stress. However, this applies not to the frontalis muscle, which seems not to respond to the present mental stress paradigm. In particular, the post hoc analysis showed elevated muscle activity of the trapezius muscle in the higher neuroticism group during the expectation of the first electrical shock, which may point towards a more unambiguous muscle response when neuroticism is considered as a personality dimension in the analysis. The personality dimension of neuroticism should be considered, especially in future studies that use sEMG of the trapezius muscle to investigate the anticipation of stressors, as this might allow for a more differentiated and objective risk assessment of mental stress in the workplace.
The authors would also like to gratefully acknowledge Timon Voll, Felix Epple, and Falko Riempp for help carrying out the measurements.
References
(2013). Personality factors and suicide risk in a representative sample of the German general population. PLoS One, 8(10), Article e76646. https://doi.org/10.1371/journal.pone.0076646
(2018). Recognition of a response to potential threat: Evidence for a facial expression of anxiety? Psychology & Neuroscience, 11(1), 18–27. https://doi.org/10.1037/pne0000116
(2012). Acute psychosocial stress: Does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology, 37(8), 1111–1134. https://doi.org/10.1016/j.psyneuen.2011.12.010
(2018). Occupational exposure factors for mental and behavioral disorders at work: The FOREC thesaurus. PLoS One, 13(6), Article e0198719. https://doi.org/10.1371/journal.pone.0198719
(2016). Eyebrow emotional expression recognition using surface EMG signals (vol 168, pg 871, 2015). Neurocomputing, 177, 671. https://doi.org/10.1016/j.neucom.2015.12.059
(2010). Cardiovascular, hormonal, and emotional responses to the TSST in relation to sex and menstrual cycle phase. Psychophysiology, 47(3), 550–559. https://doi.org/10.1111/j.1469-8986.2009.00961.x
(1987). Neuroticism, somatic complaints, and disease: Is the bark worse than the bite? Journal of Personality, 55(2), 299–316. https://doi.org/10.1111/j.1467-6494.1987.tb00438.x
(2001). Gender differences in personality traits across cultures: Robust and surprising findings. Journal of Personality and Social Psychology, 81(2), 322–331. https://doi.org/10.1037/0022-3514.81.2.322
(2013). Effects of viewing affective pictures on sEMG activity of masticatory and postural muscles. Neuroscience Letters, 544, 10–14. https://doi.org/10.1016/j.neulet.2013.02.053
(2016). Subjective mental workload and its correlation with musculoskeletal disorders in bank staff. Journal of Manipulative and Physiological Therapeutics, 39(6), 420–426. https://doi.org/10.1016/j.jmpt.2016.05.003
(2018). What is stress? A systems perspective. Integrative and Comparative Biology, 58(6), 1019–1032. https://doi.org/10.1093/icb/icy114
(2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–391. https://doi.org/10.1037/0033-2909.130.3.355
(2013). The effects of workplace stressors on muscle activity in the neck-shoulder and forearm muscles during computer work: A systematic review and meta-analysis. European Journal of Applied Physiology, 113(12), 2897–2912. https://doi.org/10.1007/s00421-013-2602-2
(1979). Facial expressions of emotion. Annual Review of Psychology, 30, 527–554. https://doi.org/10.1146/annurev.ps.30.020179.002523
(2017). Mental stress and human cardiovascular disease. Neuroscience & Biobehavioral Reviews, 74(Pt B), 269–276. https://doi.org/10.1016/j.neubiorev.2016.10.011
(2017). The effects of forearm support and shoulder posture on upper trapezius and anterior deltoid activity. The Journal of Physical Therapy Science, 29(5), 793–798. https://doi.org/10.1589/jpts.29.793
(2003). A very brief measure of the Big-Five personality domains. Journal of Research in Personality, 37(6), 504–528. https://doi.org/10.1016/S0092-6566(03)00046-1
(2004). Anxious responses to predictable and unpredictable aversive events. Behavioral Neuroscience, 118(5), 916–924. https://doi.org/10.1037/0735-7044.118.5.916
(2013). Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nature Reviews Neuroscience, 14(7), 488–501. https://doi.org/10.1038/nrn3524
(2017). Can work make you mentally ill? A systematic meta-review of work-related risk factors for common mental health problems. Occupational and Environmental Medicine, 74(4), 301–310. https://doi.org/10.1136/oemed-2016-104015
(2011). Individual differences in adaptation of cardiovascular responses to stress. Biological Psychology, 86(2), 129–136. https://doi.org/10.1016/j.biopsycho.2010.03.015
(2016). Sex hormones adjust “sex-specific” reactive and diurnal cortisol profiles. Psychoneuroendocrinology, 63, 282–290. https://doi.org/10.1016/j.psyneuen.2015.10.012
(2004). The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. American Journal of Psychiatry, 161(4), 631–636. https://doi.org/10.1176/appi.ajp.161.4.631
(1993). The “Trier Social Stress Test” – a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. https://doi.org/10.1159/000119004
(2004). Consistency in physiological stress responses and electromyographic activity during induced stress exposure in women and men. Integrative Psychological and Behavioral Science, 39(2), 105–118. https://doi.org/10.1007/bf02734276
(2009). Public health significance of neuroticism. American Psychologist, 64(4), 241–256. https://doi.org/10.1037/a0015309
(2019). The altered somatic brain network in state anxiety. Frontiers in Psychiatry, 10, Article 465. https://doi.org/10.3389/fpsyt.2019.00465
(2018). NASA-TLX assessment of surgeon workload variation across specialties. Annals of Surgery, 271(4), 686–692. https://doi.org/10.1097/SLA.0000000000003058
(2014). Experimentally induced stress validated by EMG activity. PLoS One, 9(4), Article e95215. https://doi.org/10.1371/journal.pone.0095215
(2016). Impact of early life adversity on EMG stress reactivity of the trapezius muscle. Medicine (Baltimore), 95(39), Article e4745. https://doi.org/10.1097/MD.0000000000004745
(2002). Psychophysiology of work: Stress, gender, endocrine response, and work-related upper extremity disorders. American Journal of Industrial Medicine, 41(5), 383–392. https://doi.org/10.1002/ajim.10038
(2002). Effects of experimentally induced mental and physical stress on motor unit recruitment in the trapezius muscle. Work and Stress, 16(2), 166–178. https://doi.org/10.1080/02678370210136699
(1994). Psychophysiological stress and EMG activity of the trapezius muscle. International Journal of Behavioral Medicine, 1(4), 354–370. https://doi.org/10.1207/s15327558ijbm0104_5
(2001). High and low neuroticism predict different cortisol responses to the combined dexamethasone – CRH Test. Biological Psychiatry, 49(5), 410–415. https://doi.org/10.1016/s0006-3223(00)01056-8
(2007). Autonomic and muscular responses and recovery to one-hour laboratory mental stress in healthy subjects. BMC Musculoskeletal Disorders, 8, Article 81. https://doi.org/10.1186/1471-2474-8-81
(2017). How age, sex and genotype shape the stress response. Neurobiology of Stress, 6, 44–56. https://doi.org/10.1016/j.ynstr.2016.11.004
(2002). Associations among the Big Five, emotional responses, and coping with acute stress. Personality and Individual Differences, 32(7), 1215–1228. https://doi.org/10.1016/S0191-8869(01)00087-3
(2015). Thinking too much: Self-generated thought as the engine of neuroticism. Trends in Cognitive Sciences, 19(9), 492–498. https://doi.org/10.1016/j.tics.2015.07.003
(2012). A facial expression for anxiety. Journal of Personality and Social Psychology, 102(5), 910–924. https://doi.org/10.1037/a0026825
(2012). Neuroticism modifies psychophysiological responses to fearful films. PLoS One, 7(3), Article e32413. https://doi.org/10.1371/journal.pone.0032413
(2010). Stabilization of gaze: A relationship between ciliary muscle contraction and trapezius muscle activity. Vision Research, 50(23), 2559–2569. https://doi.org/10.1016/j.visres.2010.08.021
(2005). Stress and health: Psychological, behavioral, and biological determinants. Annual Review of Clinical Psychology, 1, 607–628. https://doi.org/10.1146/annurev.clinpsy.1.102803.144141
(2014). Big Five Inventory-SOEP (BFI-S) (Publication no. 10.6102/zis54). https://zis.gesis.org/pdfFiles/Dokumentation/Schupp+_Big_Five_Inventors_SOEP_(BFI-S).pdf
(2013). Differential effects of mental concentration and acute psychosocial stress on cervical muscle activity and posture. Journal of Electromyography and Kinesiology, 23(5), 1082–1089. https://doi.org/10.1016/j.jelekin.2013.05.009
(2017). Effects of explicit cueing and ambiguity on the anticipation and experience of a painful thermal stimulus. PLoS One, 12(8), Article e0183650. https://doi.org/10.1371/journal.pone.0183650
(2010). Cognitive activation theory of stress (CATS). Neuroscience & Biobehavioral Reviews, 34(6), 877–881. https://doi.org/10.1016/j.neubiorev.2009.03.001
(2011). Association between event-related potentials and pain ratings not as straightforward as often thought. Journal of Psychophysiology, 25(1), 18–25. https://doi.org/10.1027/0269-8803/a000028
(2013). Trapezius activity of fibromyalgia patients is enhanced in stressful situations, but is similar to healthy controls in a quiet naturalistic setting: A case-control study. BMC Musculoskeletal Disorders, 14, Article 97. https://doi.org/10.1186/1471-2474-14-97
(2012). The time course of autonomic parameters and muscle tension during recovery following a moderate cognitive stressor: Dependency on trait anxiety level. International Journal of Psychophysiology, 84(1), 51–58. https://doi.org/10.1016/j.ijpsycho.2012.01.009
(2013). Effect of experimental stress in 2 different pain conditions affecting the facial muscles. Journal of Pain, 14(5), 455–466. https://doi.org/10.1016/j.jpain.2012.12.008
(2013). Effects of visually demanding near work on trapezius muscle activity. Journal of Electromyography and Kinesiology, 23(5), 1190–1198. https://doi.org/10.1016/j.jelekin.2013.06.003



