Biomarkers of Anxiety Acquisition and Generalization in Virtual Reality Experiments
Abstract
Abstract. Anxiety disorders are characterized by exaggerated responses to a threatening situation and overgeneralization. Context conditioning has been used for the identification of risk factors. This systematic literature search identifies 16 articles published between 1990 and 2021 on differential anxiety conditioning and generalization in humans. Additionally, we provide example data for individuals suffering from panic attacks with and without depressive symptoms. Successful anxiety acquisition (discrimination between anxiety and safety context) was found on the subjective level of anxiety and US-expectancy, on the physiological level of electrodermal activity, and in the defensive behavior of startle response. Anxiety generalization (discrimination between generalization and safety context) was found on the verbal but not on the physiobehavioral level. In sum, we emphasize the impact of virtual reality on anxiety research. Verbal and physiobehavioral responses serve as reliable biomarkers for anxiety. Few studies found ratings to be the best predictor for anxiety generalization. Genetic predisposition or personality traits might foster overgeneralization.
Zusammenfassung. Angststörungen kennzeichnen übermäßige Reaktionen auf eine bedrohliche Situation und Übergeneralisierung. Zur Identifizierung von Risikofaktoren zur Entwicklung von Angststörungen wird Kontextkonditionierung angewendet. In dieser systematischen Literaturrecherche identifizieren wir 16 Artikel, die zwischen 1990 und 2021 zur differenziellen Angstkonditionierung und -generalisierung beim Menschen veröffentlicht wurden. Zusätzlich zeigen wir Beispieldaten von Personen, die an Panikattacken mit und ohne depressive Symptome leiden. Erfolgreiche Angstakquisition (Unterscheidung zwischen Angst- und Sicherheitskontext) wurde auf der subjektiven Ebene der Angst und der US-Erwartung, auf der physiologischen Ebene der elektrodermalen Aktivität und in defensivem Verhalten bei der Schreckreaktion festgestellt. Angstgeneralisierung (Unterscheidung zwischen Generalisierungs- und Sicherheitskontext) wurde auf verbaler, aber nicht auf physio-behavioraler Ebene gezeigt. Insgesamt betonen die Ergebnisse den Nutzen der Virtuellen Realität bei der Erforschung von Angst. Verbale und körperlich-verhaltensbezogene Reaktionen dienen als zuverlässige Biomarker für Angst. Nur wenige Studien fanden Ratings als besten Prädiktor für die Generalisierung von Angst. Genetische Veranlagung oder Persönlichkeitsmerkmale könnten eine Übergeneralisierung begünstigen.
Anxiety disorders affect up to 33.7 % of the population (Bandelow & Michaelis, 2015) and belong to the most frequent mental disorders (Kessler et al., 1994). Anxiety disorders implicate various disorders, some of which are associated either with fear or anxiety and others share symptoms of both (Grillon, 2008). Fear is a phasic response to a distinct threatening stimulus which, when exaggerated, is the core symptom of specific phobias (Davis et al., 2010; Mineka & Oehlberg, 2008). In contrast, anxiety is a sustained response elicited by an unpredictable threat in a potentially dangerous context (Grillon, 2008). Exaggerated anxiety responses are associated, for example, with generalized anxiety disorder (GAD) and panic disorder (PD; Bandelow & Michaelis, 2015; Bouton et al., 2001; Richter et al., 2012). These anxiety disorders share impaired safety learning (Jovanovic et al., 2012; Lissek et al., 2005) and the consequent overgeneralization of fear and anxiety responses (Dymond et al., 2015; Lissek et al., 2014).
One common behavior in anxiety patients is the avoidance of the threatening stimulus (Pittig et al., 2018). Patients report excessively high subjective fear or anxiety and exaggerated physiological and neural responses to a perceived threatening stimulus, situation, or context (for a review, see Craske et al., 2009). Exposure-based therapy is an effective treatment option for anxiety disorder patients (Norton & Price, 2007), where patients are repeatedly exposed to the feared stimulus or the anxiety-evoking context. During the intervention, patients with panic disorder, for instance, may be exposed to the stimuli they are concerned about and learn in multiple ways that their worst expectations are not met (Craske & Barlow, 2014; Pompoli et al., 2018; Sánchez-Meca et al., 2010). The newly learned association of the stimulus without any expected negative consequence inhibits the formerly created fear or anxiety memory and results in less fear or anxiety symptoms (Craske et al., 2014). However, in vivo exposure therapy has a high refusal rate (Garcia-Palacios et al., 2007; Wilson et al., 2008) and can be extremely intense, complex, impractical, and time-consuming (see Maples-Keller et al., 2017). In addition, some patients – for example, up to 27 % of PD patients (see Arch & Craske, 2009) – fail to respond to exposure therapy treatment (Craske et al., 2014; Minnen et al., 2002). The high comorbidity of anxiety disorders with other mental disorders strengthens the requirement for an adequate experimental model. PD, for instance, highly coincides with major depression disorder (MDD; Beesdo et al., 2010; Horwitz, 2010). Although PD patients showed potentiated startle responses in a threat of shock experiment, having comorbid MDD attenuated startle responses (Melzig et al., 2007). Because of the therapy interruption, the lack of response, and the return of fear and anxiety symptoms, the research aims to individuate the underlying mechanisms and implement this knowledge in the therapy of anxiety disorders to increase the patients’ acceptance and responsiveness.
Both emotional constructs, fear and anxiety, are essential for the survival of an organism. A clearly identifiable, imminent threatening stimulus elicits a phasic fear response using a specific signal (Grillon, 2008). This includes activity in the central amygdala (Alvarez et al., 2008; LeDoux, 2000), phasic body reactions for the preparation for fight or flight such as increased physiological arousal, potentiated defensive reflexes, and narrowed attention (Blanchard et al., 1993; Carlsson et al., 2006; Grillon, 2008; Koch, 1999; Mowrer & Aiken, 1954; Vuilleumier, 2005). In contrast, a threatening and potentially dangerous context elicits anxiety (Grillon, 2008) mediated by the hippocampus (Alvarez et al., 2008; Andreatta et al., 2015a; Hasler et al., 2007; Marschner et al., 2008), and the so-called extended amygdala, i. e., the bed nucleus of stria terminalis (BNST, Alvarez et al., 2011; Davis & Shi, 1999; Davis et al., 2010; Sullivan et al., 2004; Walker et al., 2003). During the expectation of a future and unpredictable threat, the overall sensory sensitivity is strongly increased (Baas et al., 2004), accompanied by a feeling of insecurity, helplessness (Grillon, 2002, 2008), an increased stress level, and the need of safety becomes frantic (Seligman, 1968; Seligman & Binik, 1977). In the laboratory, fear and anxiety in humans can be assessed on multiple affective levels involving behavioral, subjective, and physiological responses (Lang, 1995). Avoidance behavior can be assessed by asking participants to approach a stimulus or context previously associated with threat or safety (Glotzbach et al., 2012). The declarative memory of the threat and its association with a cue or a context (i. e., contingency) can be assessed by ratings (Boddez et al., 2013). In parallel, measures like the valence-dependent startle response represent the defensive behavior of an organism (Vrana et al., 1988). The arousal-dependent electrodermal activity (Glotzbach-Schoon et al., 2013a; Glotzbach-Schoon et al., 2015) indicates psychophysiological responses to a threatening cue or context. These highly developed responses of fear and anxiety to threats are crucial for the survival of an organism. However, the maladaptation of such emotional systems may cause strong suffering in individuals and the development of pathological symptoms.
Those associative learning mechanisms and their underlying processes are investigated in the laboratory by constructing conditioning experiments. Initially, associative fear and classical fear conditioning were investigated by Watson and Rayner (1920). It generally starts with a neutral stimulus (NS), which is repeatedly paired with an aversive unconditioned stimulus (US). After a few pairings (also called the acquisition of the fear response), the formerly NS becomes the conditioned stimulus (CS) as its presentation alone elicits the conditioned response (CR). In human differential fear conditioning studies, one stimulus (a threat cue, CS+) is paired with the US, whereas another stimulus (a safety cue, CS–) is never paired with the US (for a review, see Lonsdorf et al., 2017). The conditioned stimuli are presented again during subsequent extinction but without the US. In this case, a new memory trace is built, namely, the association of the CS+ without US (for a review, see Hermans et al., 2006). Both acquisition and extinction memories co-exist and may compete. The context seems to disentangle such competition and to determine which memory trace an individual expresses (Bouton, 2002, 2004; Milad et al., 2005). Patients who suffer from specific phobia show such associative learning with an extremely strong fear memory. For instance, spider phobic patients might consider a spider cue a highly threat-associated CS and subsequently show exaggerated fear responses. In a laboratory setting, cue conditioning serves as a model to investigate the acquisition and extinction processes of those specific threatening stimuli, and it allows conclusions from associative learning in healthy individuals to clinical samples. Among other brain regions, the amygdala is strongly activated in patients suffering from a specific phobia when a fear-relevant stimulus is presented (Straube et al., 2005), paralleling the strong startle potentiation to phobic material (Lang et al., 2000).
Thus, we need another model for anxiety disorders to mimic the diffuse state of anxiety and elicit a sustained fear response to unpredictable threats such as a panic attack. One model is context conditioning (Grillon, 2008). In human differential context conditioning, a context (anxiety context, CTX+) is paired with an unpredictable, aversive US, whereas another context (safety context, CTX–) is never presented with the US (Baas et al., 2004; Glotzbach-Schoon et al., 2013a). During this associative learning process, the hippocampus integrates the information of multiple single cues into one contextual representation (Fanselow, 1990; Nadel & Willner, 1980). Similarly, as in fear, anxiety responses in such paradigms can be assessed on various levels of behavior, subjective, and physiological level (Glotzbach-Schoon et al., 2013a). One challenge is the operationalization of the presentation of highly controlled contextual stimuli that are ecologically valid. Some studies presented a background color or a picture on a computer screen for several seconds (Armony & Dolan, 2001; Lonsdorf et al., 2014; Pohlack et al., 2012). The presentation of contexts in virtual reality (VR) can even increase the ecological validity, and VR can be adapted for exposure-based therapy (Baas et al., 2004; Gromer et al., 2019). Patients with anxiety disorders as well as healthy and anxious individuals tend to overgeneralize their fear and anxiety (Sep et al., 2019). Several studies investigating fear generalization found higher generalization of the fear responses, for instance, potentiated startle responses and stronger US expectancy ratings for stimuli that were similar to CS+, in high-anxious compared to low-anxious participants (Baumann et al., 2017; Dunsmoor & Paz, 2015; Dymond et al., 2015; Lissek et al., 2008b, 2014). These studies use a generalization gradient to characterize the degree of generalization (Kaczkurkin et al., 2017). In short, the less a stimulus resembles CS+, the smaller the fear response. In other words, the generalization gradient gradually decreases with decreasing similarity of a stimulus with CS+ (Lissek et al., 2008a). Linear trends indicate stronger fear generalization than quadratic trends and steeper gradients (Lissek et al., 2014).
While many studies have already focused on fear generalization, anxiety generalization has been less investigated. However, the inability of safety learning in PD patients (Lissek et al., 2009) and in GAD patients (Lissek et al., 2014) also fosters overgeneralization. Anxiety conditioning paradigms using VR technology are extremely promising because of high experimental control in an extraordinary ecological valid context (Glotzbach-Schoon et al., 2013a; Sanchez-Vives & Slater, 2005). After the acquisition of conditioned anxiety, a third context, which is the exact mixture of the anxiety and the safety context, is presented and anxiety generalization is assessed (Andreatta et al., 2015b, 2017, 2019). VR allows for various generalization contexts with any overlap with the anxiety context to assess a generalization gradient similar to the more established work on fear generalization (e. g., Lissek et al., 2014). Therefore, anxiety conditioning paradigms using VR enable the investigation of underlying mechanisms and driving factors of pathological anxiety, on the one hand, and the generalization of anxiety contexts, on the other hand. Understanding the driving factors for impaired safety learning and overgeneralization in contextual anxiety might help improve exposure-based therapy.
Goals of This Review
To date, several biomarkers for the development of pathological anxiety and generalization have been investigated in genetics as well as on the behavioral, physiological, and subjective levels. In this review, we systematically identify the biomarkers for anxiety acquisition and anxiety generalization which can be used to improve exposure-based therapy in VR in clinical populations. Considering that the findings of the reviewed studies mainly focused on healthy individuals, we additionally provide subclinical example data of anxiety generalization in participants suffering from panic attacks with or without comorbid high depression scores to test whether the identified biomarkers are supported in a subclinical population.
Methods
The systematic literature search was conducted according to PRISMA guidelines (Moher et al., 2009; Moher et al., 2015). The search was carried out on the Web of Science. We considered articles published between 1990 and 21 April 2021 and written in English.
The terms for the search included: (“virtual reality” OR “VR” OR “virtual” OR “virtual environment” OR “virtual reality environment”) AND (“classical conditioning” OR “fear conditioning” OR “context conditioning” OR “associative learning” OR “sustained fear” OR “contextual fear” OR “contextual fear conditioning” OR “conditioned anxiety” OR “NPU” OR “anxiety extinction” OR “anxiety generalization” OR “generalization processes”).
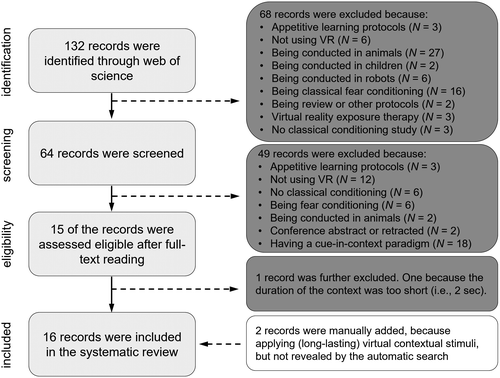
The search on the Web of Science returned 132 articles, which were screened based on their abstract (see Figure 1). To be considered in this systematic review, the studies had to meet the following criteria: (1) being conducted in humans; (2) having used VR; (3) presenting an aversive learning protocol, meaning that the US was an aversive or threatening event; (4) not being a fear or cue conditioning protocols; and (5) not being a review.
Sixty-four articles remained eligible and were read to screen them further. After this screening, 15 records were declared eligible, but we decided to exclude one study (Stolz et al., 2019) because the duration of the context was rather short. Finally, we manually included two additional studies (Andreatta et al., 2015b; Molet et al., 2013) as they used long-lasting virtual stimuli but had not been recognized by the automatic search. Altogether, we considered 16 articles (see Figure 1). Table 1 indicates the experimental settings of the studies.

Example Data
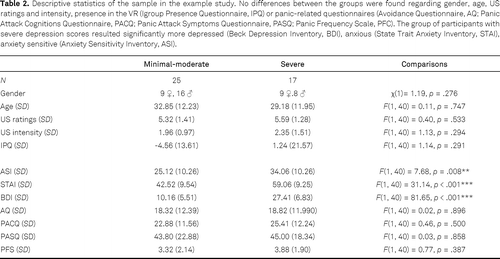
Our example data consisted of 42 participants (Table 2) who reported at least one panic attack (PA) in the last 2 weeks as reported in the Panic Frequency Scale (PFS, included in the Comprehensive Panic Profile; Clum et al., 1995). Participants were recruited via advertisements in local journals. Seventeen participants scored 20 or higher on the Becks Depression Inventory (BDI-II, Hautzinger et al., 2006) and were assigned to the severe depressive symptoms group, and 25 participants scored lower than 20 and were assigned to the minimal / moderate depressive symptoms group. Participants completed the Comprehensive Panic Profile (Clum et al., 1990) including the subscales Avoidance Questionnaire (AQ), Panic Attack Cognitions Questionnaire (PACQ), and Panic Attack Symptoms Questionnaire (PASQ). Additionally, they filled in the State Trait Anxiety Inventory (STAI, Laux et al., 1981), the Anxiety Sensitivity Inventory (ASI, Alpers and Pauli, 2001), and the Igroup Presence Questionnaire (IPQ, Schubert et al., 2001). All participants underwent an anxiety conditioning procedure in virtual reality. Thereby, they perceived unpredictable, mildly painful electric stimuli on the inner forearm when guided through one (anxiety context) but not another context (safety context). In a subsequent generalization test phase, participants were additionally guided through a third context (generalization context), which was the exact mix of the anxiety and the safety context. Anxiety and US expectancy ratings were assessed on a 100-point visual analog scale ranging from 0 (no anxious, no association) to 100 (very anxious, perfect association). Startle responses were assessed in all contexts and in the corridor (ITI), which connected all contexts. After data preprocessing (see Blumenthal et al., 2005), we calculated the difference raw scores from the respective contexts and the ITI to test the interindividual differences (see also Lonsdorf & Merz, 2017).
Overview of the Findings
Considering that the prerequisite for the generalization of a learned response is to learn that response, we divide the findings into the acquisition phase and the generalization phase. Discriminative defensive responses are depicted in Figure 2.

Anxiety Acquisition
The investigation of biomarkers on anxiety learning in healthy individuals as well as in clinical samples requires a model that reliably induces anxiety under highly controlled conditions. Thereby, anxiety acquisition is seen as successful when individuals show differential responses between CTX+ and CTX–. In this case, one can assume that CTX+ is associated with unpredictable threats, whereas the safety context predicts no threat, i. e., safety. This differentiation is commonly measured on various levels like the behavioral, physiological, and subjective levels.
Three studies (Glotzbach et al., 2012; Houtekamer et al., 2020; Molet et al., 2013) tested behavioral avoidance in a VR paradigm after anxiety acquisition, and the results were mixed. Glotzbach et al. (2012) and the second experiment by Molet et al. (2013) found avoidance behavior, as participants decided to enter a neutral and / or safety context more often than the anxiety / aversive context, meaning they avoided entering the anxiety / aversive context. In contrast, Houtekamer et al. (2020) found that participants had no preference for entering a context after anxiety acquisition. The discrepancy in the findings can be explained by the presence of a searching task in Houtekamer et al. (2020) but not in Glotzbach et al. (2012) and Molet et al. (2013). However, the free choice of navigation through VR contexts under high experimental control makes it possible to investigate contextual avoidance behavior in anxiety conditioning experiments.
In anxiety conditioning, valence (positive vs. negative), arousal (intensity), and anxiety ratings of the virtual contexts were frequently assessed together with the individuals’ US expectancy ratings. Most studies opted to collect the ratings at the end of each experimental block to prevent confounding effects induced by cognitive processes on the physiological and behavioral variables (Tabbert et al., 2006). In detail, after the last anxiety acquisition block, the 12 anxiety conditioning studies that investigated subjective anxiety reported stronger subjective anxiety in CTX+ compared to CTX– (Andreatta et al., 2015a, 2015b, 2017, 2019, 2020; Genheimer et al., 2017; Glotzbach-Schoon et al., 2015, 2013b, 2013c; Glotzbach et al., 2012; Neueder et al., 2019; Tröger et al., 2012). Interestingly, results were independent of the reinforcement rate of the US, which ranged between 60 % and 100 %. US-expectancy ratings revealed successful anxiety acquisition, i. e., higher expectancy of the US was indicated in CTX+ compared to CTX–, in all 12 studies that reported US expectancy ratings (Andreatta et al., 2015a, 2015b, 2017, 2019, 2020; Genheimer et al., 2017; Glotzbach-Schoon et al., 2013b, 2013c, 2015; Glotzbach et al., 2012; Kroes et al., 2017; Tröger et al., 2012).
Reports on the instructions of contingencies between the US and the contexts were very inhomogeneous (see Table 1), ranging from fully instructed about the context in which the US was delivered to a vague and general instruction that the aversive US might come any time. Despite the heterogeneity of the instructions, the results of all reported studies indicate that anxiety conditioning worked on a subjective level.
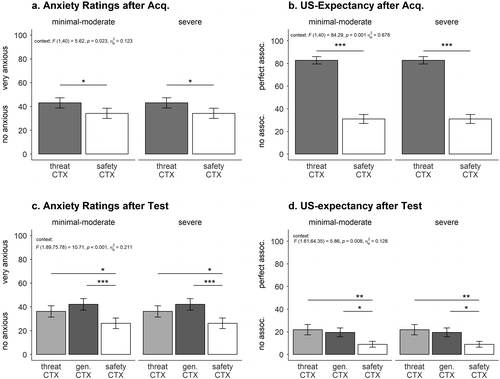
This suggests that, on the subjective level, conditioned anxiety can be acquired independently from the methodological settings. In the same vein, high anxious individuals might easily acquire and then report feeling threatened and anxious in specific contexts. This means they might not necessitate strong (e. g., clear instructions) or numerous (e. g., with a 100 % CTX–US contingency) associations between the feared event (e. g., the panic attack) and the conditioned context (e. g., the shopping mall) to report anxiety. Further, our example of individuals suffering from panic attacks demonstrates the clear acquisition of verbal conditioned anxiety (i. e., anxiety ratings and US expectancy); interestingly, this was independent of their level of depression (see Figure 3a and Figure 3b).
The effects and findings differ for physiological measurements. Electrodermal activity is a measure of physiological arousal and reflects the preparation level for a defensive response (Lang et al., 2000; LeDoux, 2003). Because it refers to contextual paradigms, this response has the advantage of disentangling the long-lasting and sustained arousal elicited by a threatening context as indicated by the skin conductance level (SCL) and the short-lasting and phasic arousal elicited by a threatening cue indicated by the skin conductance response (SCR; Boucsein et al., 2012). Altogether, ten studies reported skin conductance during anxiety acquisition, and all of them found stronger electrodermal activity, i. e., SCL, in CTX+ compared to CTX– at least at the end of acquisition (Alvarez et al., 2008; Andreatta et al., 2015b, 2017, 2020; Glotzbach-Schoon et al., 2013b, 2013c, 2015; Kroes et al., 2017; Neueder et al., 2019; Tröger et al., 2012). Kroes et al. (2017) report context discrimination in skin conductance only after exploratory post-hoc tests without correction for multiple testing.
Additionally, the startle response serves as an indicator of behavioral valence and affect and is assumed to be potentiated by aversive and threatening stimuli compared to neutral and nonthreatening stimuli (Hamm et al., 1993). Twelve of our reviewed studies used the startle response to measure defensive reaction during anxiety acquisition. Potentiated startle responses in CTX+ compared to CTX– at least in late acquisition were found in ten studies (Andreatta et al., 2015b, 2017, 2019; Genheimer et al., 2017; Glotzbach-Schoon et al., 2013c, 2015; Houtekamer et al., 2020; Kroes et al., 2017; Neueder et al., 2019; Tröger et al., 2012). The reinforcement rate also seems to be a remarkable factor in the success of anxiety acquisition. Houtekamer et al. (2020) reinforced 60 % of their CTX+ trials and analyzed only a selection of participants that discriminated the contexts regarding the startle response. Along the same line, Genheimer et al. (2017) used a reinforcement rate of 80 % and reported context discrimination in startle responses only at the end of the acquisition phase. Therefore, it seems that the reinforcement rate mirrors the strength of the CTX–US association in the startle response. These findings are also supported by our subclinical example showing significantly stronger conditioned anxiety on the startle level in the threatening context than in the safety context (see Figure 4a). Apparently, the high contingency rate (i. e., 100 %) between the threatening context and the aversive US ruled out individual differences (Lissek et al., 2006) as such a discriminative response was evident no matter whether participants presented high depressive scores or not.
Interestingly, two studies (Andreatta et al., 2020; Glotzbach-Schoon et al., 2013c) found opposing results when investigating anxiety sensitivity or trait anxiety. The former found a delayed acquisition of conditioned anxiety at the startle level, which seemed to be driven by participants’ anxiety sensitivity: The more anxiety sensitive these were, the less discrimination between the context was revealed at the startle level (Andreatta et al., 2020). The latter found (marginal) quicker context discrimination in high-anxious individuals, meaning startle potentiation in the anxiety context as compared to the safety context already during the first acquisition phase (Glotzbach-Schoon et al., 2013c). Such contrasting effects in these two studies might be related to the distinct aspects of anxiety investigated. Indeed, anxiety sensitivity refers to sensitivity toward physical, psychological, and social consequences (Reiss et al., 1986), while trait anxiety refers to a broader and more general anxious feeling toward threatening and stressful situations (Clark et al., 1994).
Altogether, both physiological arousal (i. e., electrodermal activity) and defensive responses (i. e., startle reflex) were reliable measurements for predicting the success of associative learning. Different from the subjective indices, behavioral and physiological measurements seemed more sensitive to the learning protocol.
In summary, the existing literature and the example data provided demonstrate that VR can be a reliable tool for acquiring conditioned anxiety. Aversive ratings and physiobehavioral measures of startle and skin conductance responses confirm strong defensive responses on both the explicit and the implicit level. The study of acquired anxiety and its underlying mechanisms is indispensable to better understand why some but not other individuals have a higher risk of developing pathological anxiety and which mechanisms should be tackled during the therapy. For instance, knowing that the verbal reports of a patient do not necessarily depend on how often or how explicit an aversive event happens in one context suggests that, in therapy, the simple exposure to the threatening context might not be enough to reduce the subjective anxiety. In comparison, knowing that physiobehavioral reactions of a patient are more sensitive to the frequency of the association between an aversive event and a context suggests that in therapy the amount of exposure to the threatening context should be larger than the associative events for efficiently reduced automatic anxiety.
Anxiety Generalization
Five studies (see Table 1) investigated generalization mechanisms of conditioned anxiety (Andreatta et al., <litr=5>2015b, 2017, 2019, 2020; Neueder et al., 2019). All studies used virtual reality and reported successful anxiety acquisition, i. e., discrimination between CTX+ and CTX–.
Generalization is an adaptive mechanism as it allows a prompt response to novel contexts based on previous experiences in similar but different contexts (Dymond et al., 2015). In other words, if we learned that crossing the highway can be dangerous, it can be adaptive to generalize this knowledge to the rail tracks, whereas generalizing this knowledge to circle paths (i. e., overgeneralization) can become quite debilitating. It remains unclear why some individuals adaptively generalize while others overgeneralize. A better understanding of the underlying and the cognitive mechanisms that cause us to generalize can greatly contribute to tackling the pathological mechanisms in therapy. Moreover, investigating which factors may facilitate (over–)generalization gives further insight into the target mechanisms.
Generalizing a conditioned response is based on two processes (Dymond et al., 2015): On the one hand, the stimuli that elicit a generalized response share conceptual characteristics with the threatening object or context (semantic generalization); on the other hand, the stimuli that elicit a generalized response share physical properties with the threatening object or context (perceptual generalization). All of the studies we found investigated perceptual generalization. Specifically, the so-called generalization context (G-CTX) was an office that shares features (i. e., furniture) of the anxiety and the safety context. Most studies used one generalization context, which consisted of 50 % CTX+ features and 50 % CTX– features. If individuals respond to the G-CTX similar to CTX+, one can conclude that they were generalizing anxiety to the novel context. The idea is that individuals with pathological fear or anxiety responses tend to overrate the memory trace associated with the threatening context and consequently show stronger defensive responses. One advanced study (Andreatta et al., 2020) included a stimulus gradient similar to classical fear generalization paradigms (Lissek et al., 2008a). Besides a generalization context consisting of half CTX+ and half CTX–, two additional contexts were used, one more similar to CTX+ (75 % features of CTX+, 25 % features of CTX–) and one more similar to CTX– (25 % features of CTX+, 75 % features of CTX–).
On a subjective level, all studies found higher anxiety ratings and US expectancy for CTX+ and G-CTX compared to CTX–; these effects did not change over time (Andreatta et al., 2015b, 2017, 2019, 2020; Neueder et al., 2019). The subjective anxiety responses seem to follow the strategy “better safe than sorry,” meaning that individuals prefer to feel threatened and to expect the threat within a context that shares half of the physical properties with the threatening context. This conditioned anxiety memory seems stable over time: Even up to 2 weeks after anxiety acquisition, participants discriminated CTX– and CTX+ and reported comparable ratings for G-CTX as for CTX+ (Andreatta et al., 2017). Andreatta et al. (2020) extended these findings and demonstrated that the subjective anxiety responses were generalized in the 50 % G–CTX as well as in the 75 % G-CTX but not to the context that shared only 25 % of the features with CTX+. This adaptive lack of generalization for a context that is mainly related to the safety context is even more relevant if one considers that in this study (only) the US was delivered in half of the trials to prevent the extinction of the conditioned response.
Electrodermal activity did not differ between anxiety and the safety context or the generalization contexts, when no US was delivered during the generalization phase (Andreatta et al., 2015b, 2017, 2019; Neueder et al., 2019), suggesting that the physiological arousal extinguished. When contexts are shown repeatedly without US, a novel memory trace (i. e., CTX–no-US) is formed, and gradually the conditioned response is reduced until it disappears. This learning is called extinction (Milad & Quirk, 2012; Quirk & Mueller, 2008). In contrast, the partial reinforcement during the generalization phase applied by Andreatta et al. (2020) revealed a larger skin conductance level in CTX+ compared to CTX–, suggesting that the physiological arousal did not extinguish because the aversive event (the US) was still being delivered. Another explanation of these results might be habituation effects. Strong habituation characterizes electrodermal activity, i. e., physiological arousal decreases over time (Boucsein et al., 2012). The habituation hypothesis seems to be supported by the relatively long duration of the one-day protocols (Andreatta et al., 2015b, 2019; Neueder et al., 2019), whereas Andreatta and colleagues (2020) reduced the duration of each experimental session as the acquisition, and the generalization phase were split over two different days. Physiological arousal was not generalized to the novel generalization contexts (Andreatta et al., 2020). It is possible that different mechanisms underlie the generalization of sustained fear and the generalization of phasic fear. Therefore, we think physiological responses rely on the elements within the context (i. e., the furniture) rather than on a conjunctive concept of the context (Rudy, 2009). Consequently, we cannot detect the generalization of the physiological responses on a group level.
The defensive responses measured during the generalization phase showed potentiation of the startle response in CTX+ compared to CTX– in all studies, suggesting a stronger conditioned anxiety response. In contrast, our subclinical sample revealed potentiated startle responses in CTX– compared to CTX+ during the generalization test phase but only for individuals with minimal or moderate depression scores (see Figure 4b). Although the sample size is relatively small and therefore the findings should be taken with caution, we think these findings align with those of the acquisition phase and the current literature for two reasons: First, interindividual differences may become more evident during the generalization phase as there is no obvious association with the threat as it was during acquisition (Lissek et al., 2006). Such a less obvious association between the threat and the anxiety context might have facilitated the generalization of the conditioned anxiety responses in the other context visited during acquisition (i. e., the safety context). Second, severe depressive scores blunt discriminative startle responses (Melzig et al., 2007), and individuals with severe depressive scores in our example could not discriminate across the three contexts.
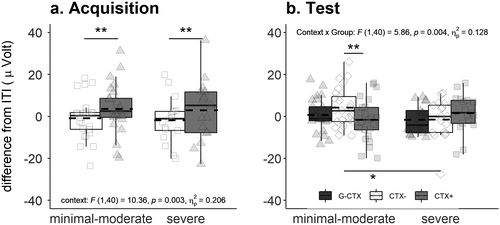
In healthy individuals, startle response was not potentiated in any generalization context as no difference was found in CTX–, suggesting that the G-CTX did not elicit anxiety responses. Most studies used the 50 % generalization context, and it is conceivable that, in healthy individuals, the safety-associated furniture in the generalization context might have been more salient than the threat-associated furniture and consequently led to reduced startle responses (Andreatta et al., 2015b, 2017, 2019; Neueder et al., 2019). Interestingly, startle responses were not potentiated in the generalization context, which shared 75 % of the furniture with the anxiety context (Andreatta et al., 2020). Despite the possibility that the safety-related furniture in this context was sufficient for inhibiting defensive responses, it is also conceivable that the startle response is not the most appropriate dependent variable for measuring sustained fear responses. In other words, startle responses are elicited by short and phasic stimuli (Blumenthal et al., 2005), which were randomly presented during the context visit. The startle response depends on when and where the phasic startle-eliciting probes were presented and cannot detect the perception of the whole context. One study demonstrated that conditioned anxiety responses (i. e., startle response) were comparable across the contexts 2 weeks after anxiety acquisition, suggesting facilitated anxiety generalization (Andreatta et al., 2017). Conceivably and in line with animal studies (Biedenkapp & Rudy, 2007), the memory trace acquired during anxiety acquisition for CTX+ and CTX– gradually decayed over time and became weaker (Frankland & Bontempi, 2005). Consequently, participants generalized their responses more.
Three studies included interindividual differences like gene variants (Andreatta et al., 2019), anxiety sensitivity (Andreatta et al., 2020), and panic attacks (Neueder et al., 2019). Altogether, these studies demonstrated facilitated the generalization of conditioned anxiety in those participants at risk for anxiety disorder, such as carriers for the met-allele of the brain-derived neurotrophic factor gene (BDNF; Andreatta et al., 2019), high-anxiety sensitive individuals (Andreatta et al., 2020), and individuals suffering from panic attacks (Neueder et al., 2019). Distinctly, the overgeneralization of conditioned anxiety was mediated by anxiety sensitivity and panic attacks on the verbal level, albeit by a genetic variation on the startle response. Our example data support such evident generalization of verbal conditioned anxiety. Thus, participants suffering from panic attacks demonstrated comparable ratings for G-CTX and CTX+ but higher than for CTX– (see Figures 3c and 3d). Again, having high depressive scores does not seem to modulate verbal responses. Considering that the interaction between genetic factors and life experience is crucial for the etiology and development of anxiety disorders (Ressler, 2020; Schiele & Domschke, 2018), it is striking that, thus far, no study has investigated how the gene x environment interaction mediates generalization processes.
In summary, the existing anxiety generalization literature is still very sparse, and few studies have been conducted. Nevertheless, results show a generalization of conditioned anxiety in healthy individuals which seems mediated by time and interindividual differences. The implementation of gradually changing contexts using VR technology enables the investigation of anxiety generalization processes similar to the already more established fear generalization processes. Future studies could try to compare the generalization gradient of conditioned anxiety with the generalization of conditioned fear.
Conclusions
The current review aimed to identify biomarkers for experimental anxiety acquisition and their impact on generalization processes. In general, conditioned anxiety responses were successfully acquired on all analyzed dependent variables, but distinct aspects influenced these. First, discriminative responses were evident in all studies no matter how strong (i. e., CTX–US contingency) and how explicit (i. e., instruction) the association was between a context and the threatening event. In contrast, physiobehavioral responses were more sensitive to the CTX–US contingency and the instructions than the verbal responses. Second, the generalization of conditioned anxiety was evident on the verbal level but not on the physiobehavioral level. Third, risk factors for anxiety disorder, such as genetic (Montag et al., 2010) and personality traits (Sandi & Richter-Levin, 2009), seem to facilitate anxiety generalization, which in turn might be the predisposition mechanism of these disorders (Sep et al., 2019).
Similar to anxiety patients, distinct processes seem to underlie verbal and physiological responses in healthy or subclinical individuals (LeDoux & Pine, 2016). Knowing which factors are crucial for modulating one or the other response level may contribute to improving treatments (Craske et al., 2018). Considering the crucial role of instructions in modulating conditioned fear responses (Hamm & Weike, 2005; Mertens et al., 2016), we strongly suggest better reporting for instructions in future studies. Moreover, the studies of this systematic review indicate no modulation of the ratings but an unclear role of instruction for physiological conditioned anxiety. It would be interesting to investigate the role of explicit or verbal knowledge in the etiology of anxiety-related disorders paralleling the findings on instructed fear, for example, as a driving mechanism for specific phobia, such as spider phobia (Fyer, 1998; Olsson & Phelps, 2007).
This systematic review showed that paradigms in VR have an enormous potential to revolutionize anxiety research to a more realistic and reliable threat experience under well-controlled conditions. The revealed interindividual differences, for example, in genetics (Andreatta et al., 2019; Glotzbach-Schoon et al., 2013b), anxiety sensitivity (Andreatta et al., 2020), and trait anxiety (Glotzbach-Schoon et al., 2013c), demonstrated that virtual paradigms presenting unpredictable threats can detect subclinical mechanisms involved in the etiology and maintenance of anxiety disorders (Mineka & Oehlberg, 2008).
Based on the literature on healthy individuals, suggestions to improve VRET in clinical populations should be highlighted. Importantly, successful anxiety acquisition could be confirmed reliably in VR experiments on a subjective level, i. e., higher aversive ratings and US expectancy in CTX+ compared to CTX–, and on psychobehavioral and physiological levels, i. e., potentiated startle responses and higher electrodermal activity in CTX+ compared to CTX–. Possibilities of VR technology could still be more exhaustive, for instance, by analyzing avoidance behavior. On this basis, anxiety research can take the next step and transfer the research to clinical samples, especially anxiety disorders like GAD or PD. Craske and colleagues (2014) suggested several strategies to maximize exposure therapy based on fear-extinction studies. This review may be a good platform for implementing these suggestions. For instance, two suggested strategies are to violate patients’ expectations and use multiple contexts during extinction. With VR, we can combine these strategies. Violation of a patient’s expectations can be achieved by being in a threat-similar situation as anxiety (or fear) memories are evoked but no threat is delivered. Parallel to that, the patient is exposed to multiple contexts with different similarity gradients. Hence, patients are trained to discriminate between the threatening context and those contexts that resemble the threatening context but are safe.
In contrast, anxiety generalization seems still underrepresented in literature. While fear generalization processes were investigated in healthy individuals as well as in clinical samples (Dunsmoor & Paz, 2015; Dymond et al., 2015; Jovanovic et al., 2012; Lissek et al., 2010, 2014), anxiety generalization is less well studied. The generalization of conditioned anxiety appears to be more complex than that of conditioned fear because for anxiety generalization, two types of representation are involved, namely, the threatening context can be perceived as a whole and as its single elements (see Rudy, 2009). The altered safety learning processes in anxiety patients compared to healthy individuals seem highly relevant in interpreting a generalization context (Lissek et al., 2009). This review shows comparable physiological responses in the generalization and safety context among healthy individuals, mainly indicated by attenuated startle responses and electrodermal activity in G-CTX compared to CTX+. In contrast, individuals generalized anxiety on subjective anxiety and US expectancy level, demonstrated by higher aversive ratings for G-CTX compared to CTX–. It remains to be investigated whether such dissociation between the physiological and verbal level is still visible or not in a clinical sample.
Until now, many studies that focused on fear generalization used a discrete stimulus and its variations to investigate generalization processes (Lissek et al., 2008b, 2014). In this way, risk factors for developing anxiety disorders like oversensitivity to threat (Shankman et al., 2013) and overgeneralization (Lissek et al., 2010) were identified. However, some anxiety disorders like generalized anxiety or panic disorders are associated with sustained rather than phasic fear (Mineka & Oehlberg, 2008). Sophisticated experimental paradigms were needed to elicit sustained threat. This review confirms the combination of context conditioning paradigms and virtual reality as an option to investigate anxiety generalization under highly controlled settings. The possibility of step-wise changes in the virtual contexts (Andreatta et al., 2020), similar to classical fear generalization paradigms, provide a very elegant tool for the more sophisticated investigations of anxiety disorders characterized by sustained fear. Moreover, VR technology and its step-wise adaptions are highly interesting for the individualization of exposure-based therapy regarding higher acceptance and therapy success for patients suffering from anxiety disorders.
Literatur
(2001). Anxiety sensitivity index. Unpublished manuscript, University of Würzburg.
(2008). Contextual fear conditioning in humans: Cortical-hippocampal and amygdala contributions. Journal of Neuroscience, 28, 6211 – 6219. https://doi.org/10.1523/JNEUROSCI.1246-08.2008
(2011). Phasic and sustained fear in humans elicits distinct patterns of brain activity. NeuroImage, 55, 389 – 400. https://doi.org/10.1016/j.neuroimage.2010.11.057
(2015a). Initial and sustained brain responses to contextual conditioned anxiety in humans. Cortex, 63, 352 – 363. https://doi.org/10.1016/j.cortex.2014.09.014
(2015b). Generalization of contextual fear in humans. Behavior Therapy, 46, 583 – 596. https://doi.org/10.1016/j.beth.2014.12.008
(2019). Human BDNF rs6265 polymorphism as a mediator for the generalization of contextual anxiety. Journal of Neuroscience Research, 97, 300 – 312. https://doi.org/10.1002/jnr.24345
(2017). Effects of context preexposure and delay until anxiety retrieval on generalization of contextual anxiety. Learning & Memory, 24, 43 – 54. https://doi.org/10.1002/jnr.24345
(2020). Generalization of conditioned contextual anxiety and the modulatory effects of anxiety sensitivity. Neurotherapeutics, 17, 1239 – 1252. https://doi.org/10.1007/s13311-020-00831-8
(2009). First-line treatment: A critical appraisal of cognitive behavioral therapy developments and alternatives. Psychiatric Clinics, 32, 525 – 547. https://doi.org/10.1016/j.psc.2009.05.001
(2001). Modulation of auditory neural responses by a visual context in human fear conditioning. Neuroreport, 12, 3407 – 3411. https://doi.org/10.1097/00001756-200110290-00051
(2004). Fear conditioning in virtual reality contexts: a new tool for the study of anxiety. Biological Psychiatry, 55, 1056 – 1060. https://doi.org/10.1016/j.biopsych.2004.02.024
(2015). Epidemiology of anxiety disorders in the 21st century. Dialogues in Clinical Neuroscience, 17, 327 – 335. https://doi.org/10.31887/DCNS.2015.17.3/bbandelow
(2017). Effects of an anxiety-specific psychometric factor on fear conditioning and fear generalization. Zeitschrift für Psychologie, 225, 200 – 213. https://doi.org/10.1027/2151-2604/a000304
(2010). Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Archives of General Psychiatry, 67, 47 – 57. https://doi.org/10.1001/archgenpsychiatry.2009.177
(2007). Context preexposure prevents forgetting of a contextual fear memory: Implication for regional changes in brain activation patterns associated with recent and remote memory tests. Learning & Memory, 14, 200 – 203. https://doi.org/10.1101/Lm.499407
(1993). Defense system psychopharmacology: An ethological approach to the pharmacology of fear and anxiety. Behavioural Brain Research, 58, 155 – 165. https://doi.org/10.1016/0166-4328(93)90100-5
(2005). Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology 42 (1), 1 – 15. https://doi.org/10.1111/j.1469-8986.2005.00271.x
(2013). Rating data are underrated: Validity of US expectancy in human fear conditioning. Journal of Behavior Therapy and Experimental Psychiatry, 44, 201 – 206. https://doi.org/10.1016/j.jbtep.2012.08.003
(2012). Publication recommendations for electrodermal measurements. Psychophysiology, 49, 1017 – 1034. https://doi.org/10.1111/j.1469-8986.2012.01384.x
(2002). Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry, 52, 976 – 986. https://doi.org/10.1016/S0006-3223(02)01546-9
(2004). Context and behavioral processes in extinction. Learning & Memory, 11, 485 – 494. https://doi.org/10.1101/lm.78804
(2001). A modern learning theory perspective on the etiology of panic disorder. Psychological Review, 108, 4 – 32. https://doi.org/10.1037//0033-295X.108.1.4
(2006). Predictability modulates the affective and sensory-discriminative neural processing of pain. NeuroImage, 32, 1804 – 1814. https://doi.org/10.1016/j.neuroimage.2006.05.027
(1994). Temperament, personality, and the mood and anxiety disorders. Journal of Abnormal Psychology, 103, 103 – 116. https://doi.org/10.1037/0021-843X.103.1.103
(1990). Validity and reliability of the panic attack symptoms and cognitions questionnaires. J Psychopathol Behav Assess, 12, 233 – 245. https://doi.org/10.1007/bf00960620
(2014). Panic disorder and agoraphobia. Clinical handbook of psychological disorders: A step-by-step treatment manual (5th ed., pp. 1 – 61). Guilford.
(2018). State-of-the-art and future directions for extinction as a translational model for fear and anxiety. Philosophical Transactions of the Royal Society B: Biological Sciences, 373, 20170025. https://doi.org/10.1098/rstb.2017.0025
(2009). Computer‐assisted delivery of cognitive behavioral therapy for anxiety disorders in primary‐care settings. Depression and Anxiety, 26, 235 – 242. https://doi.org/10.1002/da.20542
(2014). Maximizing exposure therapy: An inhibitory learning approach. Behaviour Research and Therapy, 58, 10 – 23. https://doi.org/10.1016/j.brat.2014.04.006
(1999). The extended amygdala: Are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Annals of the New York Academy of Sciences, 877, 281 – 291. https://doi.org/10.1111/j.1749-6632.1999.tb09273.x
(2010). Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology, 35, 105 – 135. https://doi.org/10.1038/npp.2009.109
(2015). Fear generalization and anxiety: Behavioral and neural mechanisms. Biological Psychiatry, 78, 336 – 343. https://doi.org/10.1016/j.biopsych.2015.04.010
(2015). Fear generalization in humans: Systematic review and implications for anxiety disorder research. Behavior Therapy, 46, 561 – 582. https://doi.org/10.1016/j.beth.2014.10.001
(1990). Factors governing one-trial contextual conditioning. Animal Learning & Behavior, 18, 264 – 270. https://doi.org/10.3758/bf03205285
(2005). The organization of recent and remote memories. Nature Reviews Neuroscience, 6, 119 – 130. https://doi.org/10.1038/nrn1607
(1998). Current approaches to etiology and pathophysiology of specific phobia. Biological Psychiatry, 44, 1295 – 1304. https://doi.org/10.1016/s0006-3223(98)00274-1
(2007). Comparing acceptance and refusal rates of virtual reality exposure vs. in vivo exposure by patients with specific phobias. Cyberpsychology & Behavior, 10, 722 – 724. https://doi.org/10.1089/cpb.2007.9962
(2017). Reinstatement of contextual conditioned anxiety in virtual reality and the effects of transcutaneous vagus nerve stimulation in humans. Scientific Reports, 7, 17886. https://doi.org/10.1038/s41598-017-18183-3
(2013a). Context conditioning in virtual reality as a model for pathological anxiety. e-Neuroforum, 4, 63 – 70. https://doi.org/10.1007/s13295-013-0047-z
(2015). Reinstatement of contextual anxiety in humans: Effects of state anxiety. International Journal of Psychophysiology, 98, 557 – 566. https://doi.org/10.1016/j.ijpsycho.2015.07.013
(2013b). Contextual fear conditioning in virtual reality is affected by 5HTTLPR and NPSR1 polymorphisms: Effects on fear-potentiated startle. Frontiers of Behavioral Neuroscience, 7. https://doi.org/10.3389/fnbeh.2013.00031
(2013c). Enhanced discrimination between threatening and safe contexts in high-anxious individuals. Biological Psychology, 93, 159 – 166. https://doi.org/10.1016/j.biopsycho.2013.01.011
(2012). Contextual fear conditioning predicts subsequent avoidance behaviour in a virtual reality environment. Cognition & Emotion, 26, 1256 – 1272. https://doi.org/10.1080/02699931.2012.656581
(2002). Associative learning deficits increase symptoms of anxiety in humans. Biological Psychiatry, 51, 851 – 858.
(2008). Models and mechanisms of anxiety: Evidence from startle studies. Psychopharmacology, 199, 421 – 437. https://doi.org/10.1007/s00213-007-1019-1
(2019). Causal interactive links between presence and fear in virtual reality height exposure. Frontiers in Psychology, 10. https://doi.org/10.3389/fpsyg.2019.00141
(1993). Emotional learning, hedonic change, and the startle probe. Journal of Abnormal Psychology, 102 (3), 453 – 465. https://doi.org/10.1037/0021-843X.102.3.453
(2005). The neuropsychology of fear learning and fear regulation. International Journal of Psychophysiology, 57, 5 – 14. https://doi.org/10.1016/j.ijpsycho.2005.01.006
(2007). Cerebral blood flow in immediate and sustained anxiety. The Journal of Neuroscience, 27, 6313 – 6319. https://doi.org/10.1523/jneurosci.5369-06.2007
(2006). Beck Depressions-Inventar (BDI-II). Harcourt Test Services.
(2006). Extinction in human fear conditioning. Biological Psychiatry, 60, 361 – 368. https://doi.org/10.1016/j.biopsych.2005.10.006
(2010). How an age of anxiety became an age of depression. The Milbank Quarterly, 88, 112 – 138.
. (2020). Investigating the efficacy of the reminder-extinction procedure to disrupt contextual threat memories in humans using immersive Virtual Reality. Scientific Reports, 10, 16991. https://doi.org/10.1038/s41598-020-73139-4
(2012). Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology, 62, 695 – 704. https://doi.org/10.1016/j.neuropharm.2011.02.023
(2017). Neural substrates of overgeneralized conditioned fear in PTSD. American Journal of Psychiatry, 174, 125 – 134. https://doi.org/10.1176/appi.ajp.2016.15121549
(1994). Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: Results from the National Comorbidity Survey. Archives of General Psychiatry, 51, 8 – 19. https://doi.org/10.1001/archpsyc.62.6.593
(1999). The neurobiology of startle. Progress in Neurobiology, 59, 107 – 128. https://doi.org/10.1016/s0301-0082(98)00098-7
(2017). Context conditioning in humans using commercially available immersive Virtual Reality. Scientific Reports, 7, 8640. https://doi.org/10.1038/s41598-017-08184-7
(1995). The emotion probe: Studies of motivation and attention. American Psychologist, 50, 372 – 385. https://doi.org/10.1037/0003-066X.50.5.372
(2000). Fear and anxiety: Animal models and human cognitive psychophysiology. Journal of Affective Disorders, 61, 137 – 159. https://doi.org/10.1016/s0165-0327(00)00343-8
(1981). STAI. State-Trait-Angstinventar. Beltz Test GmbH.
(2003). The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology, 23, 727 – 738. https://doi.org/10.1023/a:1025048802629
(2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23, 155 – 184. https://doi.org/10.1146/annurev.neuro.23.1.155
(2016). Using neuroscience to help understand fear and anxiety: A two-system framework. American Journal of Psychiatry, 173, 1083 – 1093. https://doi.org/10.1176/appi.ajp.2016.16030353
(2008a). Generalization of conditioned fear-potentiated startle in humans: Experimental validation and clinical relevance. Behaviour Research and Therapy, 46, 678 – 687. https://doi.org/10.1016/j.brat.2008.02.005
(2014). Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biological Psychiatry, 75, 909 – 915. https://doi.org/10.1016/j.biopsych.2013.07.025
(2008b). Elevated fear conditioning to socially relevant unconditioned stimuli in social anxiety disorder. The American Journal of Psychiatry, 165, 124 – 132. https://doi.org/10.1176/appi.ajp.2007.06091513
(2006). The strong situation: A potential impediment to studying the psychobiology and pharmacology of anxiety disorders. Biological Psychology, 72, 265 – 270. https://doi.org/10.1016/j.biopsycho.2005.11.004
(2005). Classical fear conditioning in the anxiety disorders: A meta-analysis. Behaviour Research and Therapy, 43, 1391 – 1424. https://doi.org/10.1016/j.brat.2004.10.007
(2010). Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. American Journal of Psychiatry, 167, 47 – 55. https://doi.org/10.1176/appi.ajp.2009.09030410
(2009). Impaired discriminative fear-conditioning resulting from elevated fear responding to learned safety cues among individuals with panic disorder. Behav Res Ther, 47, 111 – 118. https://doi.org/10.1016/j.brat.2008.10.017
(2014). Long-term expression of human contextual fear and extinction memories involves amygdala, hippocampus and ventromedial prefrontal cortex: A reinstatement study in two independent samples. Social Cognitive and Affective Neuroscience, 9, 1973 – 1983. https://doi.org/10.1093/scan/nsu018
(2017). Don’t fear ”fear conditioning”: Methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neuroscience & Biobehavioral Reviews, 77, 247 – 285. https://doi.org/10.1016/j.neubiorev.2017.02.026
(2017). More than just noise: Inter-individual differences in fear acquisition, extinction and return of fear in humans – Biological, experiential, temperamental factors, and methodological pitfalls. Neuroscience & Biobehavioral Reviews, 80, 703 – 728. https://doi.org/10.1016/j.neubiorev.2017.07.007
(2017). The use of virtual reality technology in the treatment of anxiety and other psychiatric disorders. Harvard Review of Psychiatry, 25 (3), 103 – 113. https://doi.org/10.1097/HRP.0000000000000138
(2008). Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. The Journal of Neuroscience, 28, 9030 – 9036. https://doi.org/10.1523/jneurosci.1651-08.2008
(2007). Startle reflex modulation and autonomic responding during anxious apprehension in panic disorder patients. Psychophysiology, 44, 846 – 854. https://doi.org/10.1111/j.1469-8986.2007.00560.x
(2016). Fear expression and return of fear following threat instruction with or without direct contingency experience. Cognition and Emotion, 30, 968 – 984. https://doi.org/10.1080/02699931.2015.1038219
(2005). Context modulation of memory for fear extinction in humans. Psychophysiology, 42, 456 – 464. https://doi.org/10.1111/j.1469-8986.2005.00302.x
(2012). Fear extinction as a model for translational neuroscience: Ten years of progress. Annual Review of Psychology, 63, 129 – 151. https://doi.org/10.1146/annurev.psych.121208.131631
(2008). The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychologica, 127, 567 – 580. https://doi.org/10.1016/j.actpsy.2007.11.007
(2002). Changes in PTSD patients’ narratives during prolonged exposure therapy: A replication and extension. Journal of Traumatic Stress, 15, 255 – 258. https://doi.org/10.1023/A:1015263513654
(2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6,
e1000097 . https://doi.org/10.7326/0003-4819-151-4-200908180-00135(2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews, 4, 1 – 9. https://doi.org/10.1186/2046-4053-4-1
(2013). Conditioned place preference and aversion for music in a virtual reality environment. Behavioural Processes, 92, 31 – 35. https://doi.org/10.1016/j.beproc.2012.10.001
(2010). The BDNF Val66Met polymorphism and anxiety: Support for animal knock-in studies from a genetic association study in humans. Psychiatry Research, 179, 86 – 90. https://doi.org/10.1016/j.psychres.2008.08.005
(1954). Contiguity vs. drive-reduction in conditioned fear: Temporal variations in conditioned and unconditioned stimulus. The American Journal of Psychology, 67, 26 – 38. https://doi.org/10.2307/1418068
(1980). Context and conditioning: A place for space. Physiological Psychology, 8, 218 – 228. https://doi.org/10.3758/BF03332853
(2019). Contextual fear conditioning and fear generalization in individuals with panic attacks. Front Behav Neurosci, 13,
152 . https://doi.org/10.3389/fnbeh.2019.00152(2007). A meta-analytic review of adult cognitive-behavioral treatment outcome across the anxiety disorders. The Journal of Nervous And Mental Disease, 195, 521 – 531. https://doi.org/10.1097/01.nmd.0000253843.70149.9a
(2007). Social learning of fear. Nature Neuroscience, 10, 1095 – 1102. https://doi.org/10.1038/nn1968
(2018). The role of associative fear and avoidance learning in anxiety disorders: Gaps and directions for future research. Neuroscience & Biobehavioral Reviews, 88, 117 – 140. https://doi.org/10.1016/j.neubiorev.2018.03.015
(2012). Activation of the ventral striatum during aversive contextual conditioning in humans. Biological Psychology, 91, 74 – 80. https://doi.org/10.1016/j.biopsycho.2012.04.004
(2018). Dismantling cognitive-behaviour therapy for panic disorder: A systematic review and component network meta-analysis. Psychological Medicine, 48, 1945 – 1953. https://doi.org/10.1017/S0033291717003919
(2008). Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology, 33, 56 – 72. https://doi.org/10.1038/sj.npp.1301555
(1986). Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behaviour Research and Therapy, 24, 1 – 8. https://doi.org/10.1016/0005-7967(86)90143-9
(2020). Translating across circuits and genetics toward progress in fear- and anxiety-related disorders. American Journal of Psychiatry, 177, 214 – 222. https://doi.org/10.1176/appi.ajp.2020.20010055
(2012). Dynamics of defensive reactivity in patients with panic disorder and agoraphobia: Implications for the etiology of panic disorder. Biological Psychiatry, 72, 512 – 520. https://doi.org/10.1016/j.biopsych.2012.03.035
(2009). Context representations, context functions, and the parahippocampal–hippocampal system. Learning & Memory, 16, 573 – 585. https://doi.org/10.1101/lm.1494409
(2010). Psychological treatment of panic disorder with or without agoraphobia: A meta-analysis. Clinical Psychology Review, 30, 37 – 50. https://doi.org/10.1016/j.cpr.2009.08.011
(2005). From presence to consciousness through virtual reality. Nature Reviews Neuroscience, 6, 332 – 339. https://doi.org/10.1038/nrn1651
(2009). From high anxiety trait to depression: A neurocognitive hypothesis. Trends in Neurosciences, 32, 312 – 320. https://doi.org/10.1016/j.tins.2009.02.004
(2018). Epigenetics at the crossroads between genes, environment and resilience in anxiety disorders. Genes, Brain and Behavior, 17,
e12423 . https://doi.org/10.1111/gbb.12423(2001). The experience of presence: Factor analytic insights. Presence: Teleoperators & Virtual Environments, 10, 266 – 281. https://doi.org/10.1162/105474601300343603
(1968). Chronic fear produced by unpredictable electric shock. Journal of Comparative and Physiological Psychology, 66, 402 – 411. https://doi.org/10.1037/h0026355
(1977).
The safety signal hypothesis . In H. DavisH. M. B. Hurwitz (Eds.), Operant-pavlovian interactions (pp. 165 – 187). Routledge. https://doi.org/10.4324/9781003150404(2019). The relation between anxious personality traits and fear generalization in healthy subjects: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews, 107, 320 – 328. https://doi.org/10.1016/j.neubiorev.2019.09.029
(2013). A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and / or major depressive disorder. Journal of Abnormal Psychology, 122, 322 – 338. https://doi.org/10.1037/a0030747
(2019). Threat-conditioned contexts modulate the late positive potential to faces: A mobile EEG / virtual reality study. Psychophysiology, 56,
e13308 . https://doi.org/10.1111/psyp.13308(2005). Neural mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biological Psychiatry, 59, 162 – 170. https://doi.org/10.1016/j.biopsych.2005.06.013
(2004). Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience, 128, 7 – 14. https://doi.org/10.1016/j.neuroscience.2004.06.015
(2006). Dissociation of neural responses and skin conductance reactions during fear conditioning with and without awareness of stimulus contingencies. NeuroImage, 32, 761 – 770. https://doi.org/10.1016/j.neuroimage.2006.03.038
(2012). Does pre-exposure inhibit fear context conditioning? A virtual reality study. Journal of Neural Transmission, 119, 709 – 719. https://doi.org/10.1007/s00702-011-0757-8
(1988). The startle probe response: A new measure of emotion? Journal of Abnormal Psychology, 97, 487 – 491. https://doi.org/10.1037/0021-843X.97.4.487
(2005). How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences, 9, 585 – 594. https://doi.org/10.1016/j.tics.2005.10.011
(2003). Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology, 463, 199 – 216. https://doi.org/10.1016/S0014-2999(03)01282-2
(1920). Conditioned emotional reactions. Journal of Experimental Psychology, 3, 1 – 14. https://doi.org/10.1037/h0069608
(2008). Soldier attitudes about technology-based approaches to mental health care. CyberPsychology & Behavior, 11, 767 – 769. https://doi.org/10.1089/cpb.2008.0071



